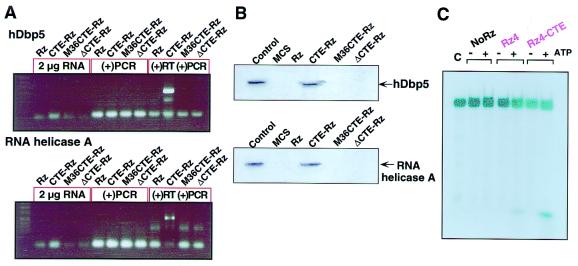
Figure 6.
Interaction in cells between tRNAVal-driven CTE-Rz and RNA helicases. (A) Coimmunoprecipitation of CTE-Rz RNA with hDbp5 and RHA (31). Either c-myc-hDbp5 (32) or HA-RHA (26, 31) was transiently cotransfected into HeLa S3 cells with the indicated versions of the TAR Rz 4 expression vectors. Immunoprecipitates obtained with the use of the appropriate tag were subjected to RT-PCR [(+)RT (+)PCR]. The CTE-Rz was detected only when wild-type CTE was present. As controls, the RNA was analyzed before RT-PCR (2 μg RNA) and after being subjected to PCR without RT treatment [(−)RT (+)PCR]. (B) RNA helicase hDbp5 and RHA interact with CTE-Rz. The indicated versions of TAR Rz4 and a multiple cloning site of pBluescript (MCS) were synthesized in vitro with the use of biotinylated UTP and then mixed with HeLa S3 cell extract from cells transfected with either c-myc-hDbp5 or HA-RHA (31). Streptavidin beads were used to precipitate the biotinylated RNAs and associated proteins. Western blotting with antibodies recognizing the appropriate tag revealed that only TAR CTE-Rz 4 interacted with the helicases. Control, whole-cell lysate from transfected cells. (C) Stimulated cleavage by CTE-Rz upon the addition of proteins precipitated with the synthesized CTE-Rz. Proteins that interact with in vitro synthesized CTE-Rz were precipitated from HeLa cells and were added to the reaction mixtures. The reaction mixtures were incubated at 37°C for 45 min in the presence or absence of 2 mM ATP and 0.2 mM GTP. Only when ATP was added to the reaction mixture was TAR CTE-Rz able to stimulate cleavage of the RNA substrate. The lesser extent of the stimulated cleavage upon the addition of the proteins and ATP was also observed, even in the case of the TAR Rz 4. It seems to be a potential enhancement by the RNA-unwinding activity of RNA helicases itself. The enhancement of cleavage by the precipitated proteins strongly suggests the presence of cellular factors responsible for the enhanced suppression by CTE-Rz.