Abstract
Electrodiagnostic testing is used widely for the full characterization of neuromuscular disorders and for providing unique information on the processes underlying the pathology of peripheral nerves and muscles. However, such testing should be considered as an extension of anamnesis and physical examination, not as pathognomonic of a specific disease entity. There are many pitfalls that could lead to erroneous interpretation of electrophysiological study results when the studies are not performed properly or if they are performed in the presence of anatomical aberrations. The diagnostic reliability of electrodiagnostic studies can be improved and the associated pitfalls overcome if the physician is familiar with all of those possible pitfalls. In this article we discuss the most common and important pitfalls associated with electrodiagnostic medicine.
Keywords: electrodiagnostic study, pitfalls, neuromuscular disorders, nerve conduction study, electromyography
Introduction
Electrodiagnostic studies, including nerve conduction studies (NCSs) and electromyography (EMG), are the gold standard for evaluating the function of peripheral nerves, neuromuscular junctions, and muscles. The main objective of electrodiagnostic studies is not only to localize but also to characterize disorders involving the peripheral nervous system. However, electrodiagnostic studies alone cannot correctly diagnose these disorders; instead they should always be considered as an extension of clinical anamnesis and physical examination. The correct interpretation of electrodiagnostic study results and application of those results clinically requires the electromyographers to have expert knowledge and experience of neuroanatomy and of peripheral disorders. They should also be familiar with the many pitfalls associated with electrodiagnostics that could lead to erroneous conclusions regarding the nature of the condition underlying peripheral nerve and muscle disorders in their patients.
In this overview we describe the various pitfalls that render it difficult to interpret the type and extent of pathophysiological changes from the results of NCSs and EMG. We include up-to-date and comprehensive information regarding commonly encountered pitfalls in electrodiagnostic studies.
Nerve Conduction Studies
After formulating differential diagnoses based on appropriate anamnesis and physical examination, NCSs are usually performed ahead of needle EMG. The technical factors defining NCS parameters are the equipment settings, patient preparation, stimulation, and interpretation of the resulting data. There are potential pitfalls associated with each of these factors (Table 1).
Table 1.
Factors affecting nerve conduction study parameters
CMAP: compound muscle action potential, NCV: nerve conduction velocity, SNAP: sensory nerve action potential.
Equipment settings
Amplifier
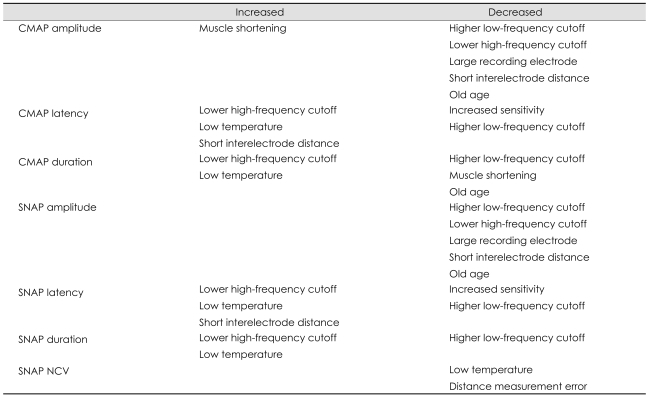
The amplifier is an important component of the equipment, which magnifies otherwise indecipherable biologic signals, altering them into conspicuous waveforms so that they can be observed and analyzed.1 With modern technology, the currently available equipment allows the examiner to review data at different sensitivities during the NCS, so that, for example, a higher level of sensitivity can be chosen to increase the precision of measurement of the onset latency of the fastest conducting fibers. A recent study2 has revealed that the sensitivity of latency measurements in diagnosing carpal tunnel syndrome is dependent upon the amplifier settings. The authors of that study presented different cutoff values for abnormalities at various amplifier settings. Hence, electromyographers should be aware that the sensitivity of the diagnosis could be altered according to the amplifier settings; that is, an increased amplifier sensitivity will result in a decreased onset latency (Fig. 1).
Fig. 1.
Amplifier gain effect. Effects of amplifier gain on the compound muscle action potential. A higher amplifier gain results in shorter onset latencies.
Filters
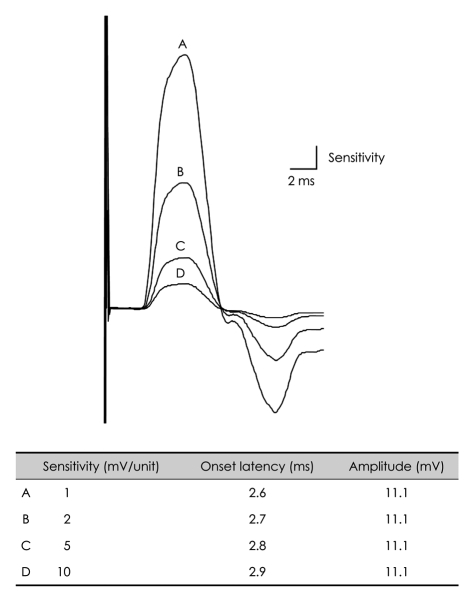
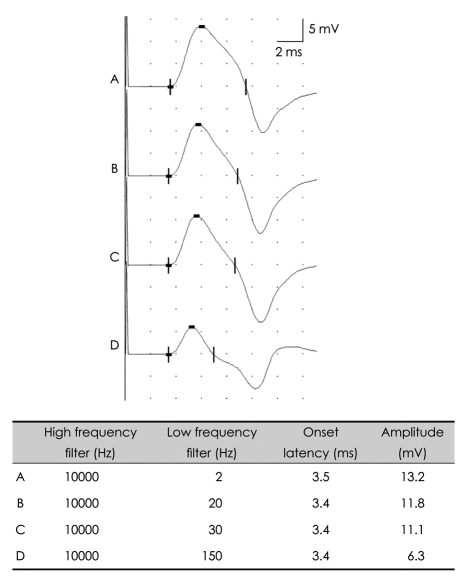
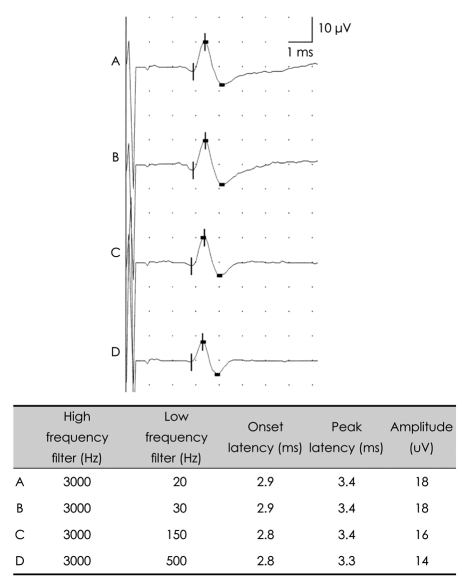
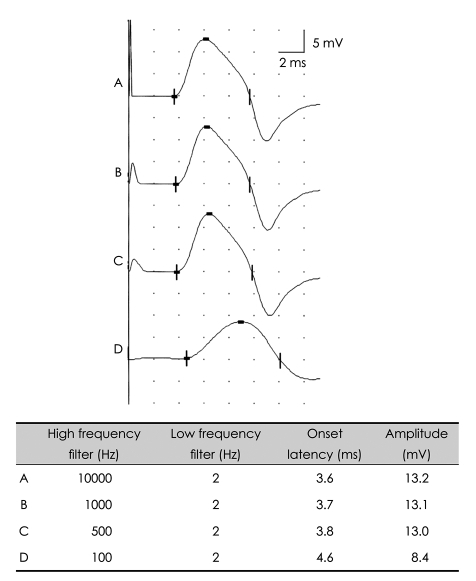
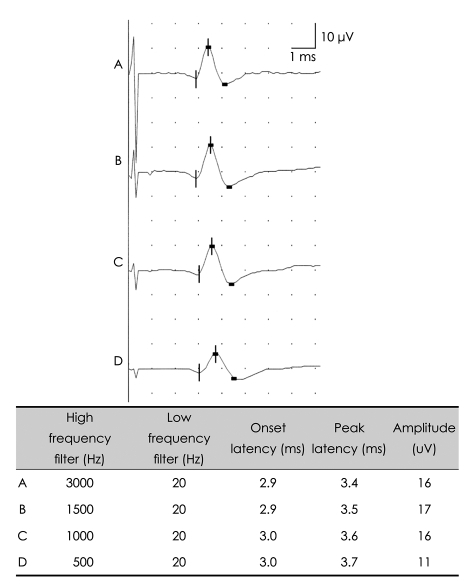
Biologic signals can be expressed as the sum of a set of simple oscillating functions (e.g., sines and cosines) whose individual phases and amplitudes summate or cancel to reproduce the original waveform.3 A filter is an electronic device that removes components at specific frequencies from the waveform.4 A high-frequency filter removes the higher frequencies, leaving the lower frequencies to pass unaffected, and is therefore commonly referred to as a lowpass filter. Likewise, a low-frequency filter removes the lower frequencies, allowing only the high frequencies to be observed, and is called a highpass filter. The recommended low-frequency cutoff for NCSs is 2-10 Hz, while the high-frequency cutoffs for sensory nerve action potentials (SNAPs) and compound action potentials (CMAPs) are 2000 and 10000 Hz, respectively.3,5 Raising the low-frequency cutoff will result in both SNAPs and CMAPs exhibiting lower-amplitude, shorter-peak latencies, with a shorter duration and greater phase accumulation, with CMAPs being affected more than SNAPs (Figs. 2 and 3). Lowering the high-frequency cutoff will result in both SNAPs and CMAPs exhibiting amplitude reduction, delayed peak/onset latency, increased duration, and smoother waveforms, with SNAPs being more affected than CMAPs (Figs. 4 and 5). Modifying the filter cutoff frequencies without understanding these basic principles could significantly distort the waveforms in the same way as if pathology were present.
Fig. 2.
Effects of low-frequency filter on the CMAP. Raising the low-frequency cutoff up to 150 Hz with a constant high-frequency cutoff produces shorter onset latencies and reduced amplitudes. CMAP: compound muscle action potential.
Fig. 3.
Effects of low-frequency filter on the sensory nerve action potential. Raising the low-frequency filter up to 150 Hz with a constant high-frequency cutoff produces shorter latencies and reduced amplitudes.
Fig. 4.
Effects of high-frequency filter on the CMAP. Lowering the high-frequency cutoff down to 500 Hz with a constant low-frequency cutoff produces prolonged onset latencies and reduced amplitudes. CMAP: compound muscle action potential.
Fig. 5.
Effects of high-frequency filter on the SNAP. Lowering the high-frequency cutoff down to 500 Hz with a constant low-frequency cutoff produces prolonged latencies and reduced amplitudes. SNAP: sensory nerve action potential.
Size of the surface electrode
In general, larger recording electrodes result in CMAPs with slightly smaller amplitudes and areas and slower conduction velocities, but no clinically significant change in onset latency or negative spike duration.3 Large electrodes are also known to reduce site-induced CMAP variability.6 Although there is a lack of consensus regarding the preferred size for surface electrodes, the most widely used in NCSs are 1 cm in diameter. However, if the muscle has only a partial lesion among a subset of motor units, CMAPs recorded using the 1-cm-diameter single electrode may not reveal the abnormal lesion, because the CMAPs represent the summation of the motor unit signals and will include only a few abnormal signals from a partial lesion. A recent study revealed the reliability and usefulness of multichannel electrodes with several small electrodes in a single-electrode platform for detecting abnormal signals.7 Ven et al.8 revealed that the SNAP amplitudes decreased with increasing size of surface recording electrodes when they are transversely positioned over the nerve.
In summary, although the optimal size of surface electrodes for NCSs has yet to be determined, it should be kept constant in a neurophysiology laboratory due to its influence on CMAP and SNAP parameters.
Patient preparation
Temperature
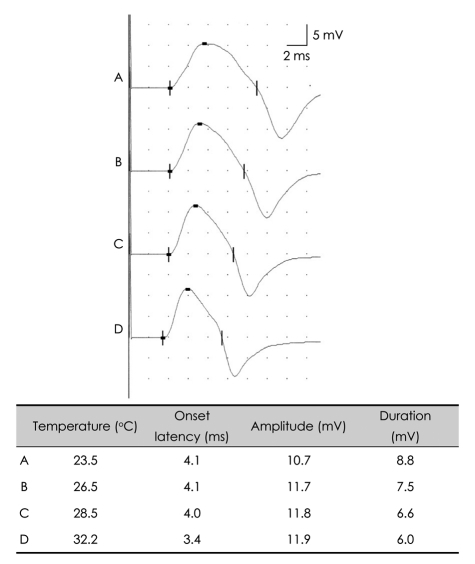
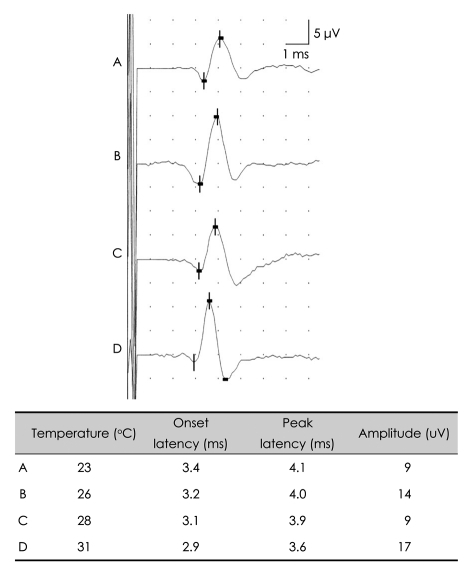
Temperature can greatly affect NCS parameters, and electromyographers should thus always check and control the temperatures of the limbs to be examined. If the upper or lower limbs are below 32° or 30°, respectively, CMAPs and SNAPs will have a prolonged duration, prolonged onset latency, a slowed nerve conduction velocity, and an increased amplitude (Figs. 6 and 7).1 Increased CMAP amplitudes caused by temperature decrements during repetitive nerve-stimulation tests could mask amplitude decrements in patients with neuromuscular junction disorders, producing false-negative results. Therefore, it is essential to control the patient's limb temperature during electrodiagnostic studies, especially when evaluate the severity of neuromuscular disease. Although the use of a hot-water bath is one of the most widely used methods for warming,9 it is difficult to control the limb temperature during recordings with this method. Instead, the use of hot-water blankets has been advocated as an alternative for controlling temperature during NCSs; it was also suggested that the limbs should be warmed for at least 25 minutes prior to commencing recording.9
Fig. 6.
Effects of limb temperature on the CMAP. Generalized cooling of the limb prolongs the latency and the duration, and slightly reduces the amplitude. CMAP: compound muscle action potential.
Fig. 7.
Effects of limb temperature on the SNAP. Generalized cooling of the limb results in shorter latencies. SNAP: sensory nerve action potential.
Electrode location
Once the patient's limbs are sufficiently warmed, surface electrodes [active (E1) and reference (E2)] are attached before stimulating the desired nerve. The examiner should be familiar with the anatomy of the nerve to be examined in order to ensure that the surface electrodes are attached appropriately.
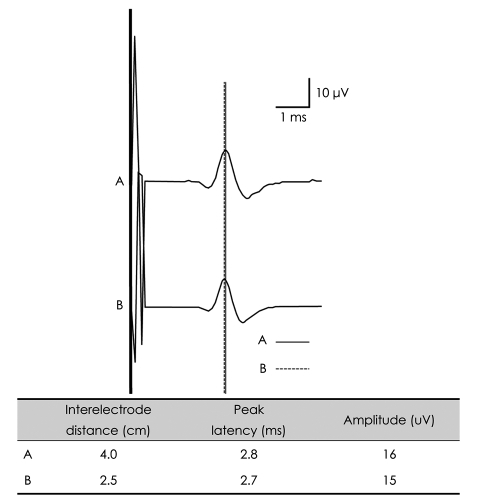
E1 is placed on the nerves or the muscles to be examined and E2 is usually placed at some distance from E1.10 Since the amplitude of signals obtained from NCSs is the difference in voltage between two electrodes [i.e., (voltage obtained from E1) - (voltage obtained from E2)], proper electrode placement is crucial for accurate waveform recording (Fig. 8).
Fig. 8.
Changes in peak latencies of the orthodromic median SNAP with varying interelectrode distances. Due to the elimination of common-mode signals, the SNAP amplitude is smaller with a shorter interelectrode distance (B) than with a longer interelectrode distance (A). The peak latency of the SNAP is shorter with a shorter interelectrode distance (B, dotted line) than with a longer interelectrode distance (A, continuous line). SNAP: sensory nerve action potential.
While recording sensory and mixed compound nerve action potentials, some authors have recommended a 4-cm separation between E1 and E2,1,4,11,12 while others13,14 have recommended a 3-cm separation. A recent paper15 suggests that the use of this narrower interelectrode separation has a negligible effect on amplitude and does not affect latency. However, this conclusion was based on studies performed on normal healthy subjects and cannot be directly applied to neuropathic patients in whom the extent of waveform distortion would be greater than in healthy subjects.
When recording CMAPs, E1 and E2 are traditionally placed over the muscle and over the corresponding tendon (belly-tendon recording), respectively. It is assumed that E2 is at an "electrically silent" position, and should not produce any electrophysiologic potentials. However, it has been shown that E2 can be electrically active.16,17 A recent study18 measured the signals recorded individually by E1 and E2 in order to assess the contribution of E2 to the signal. Significant contributions from E2 were observed, especially in the hypothenar and abductor hallucis muscles. Therefore, the measured CMAP amplitude should be interpreted with caution since it might not reflect the true pathology of the muscle under E1.
Electrode stability
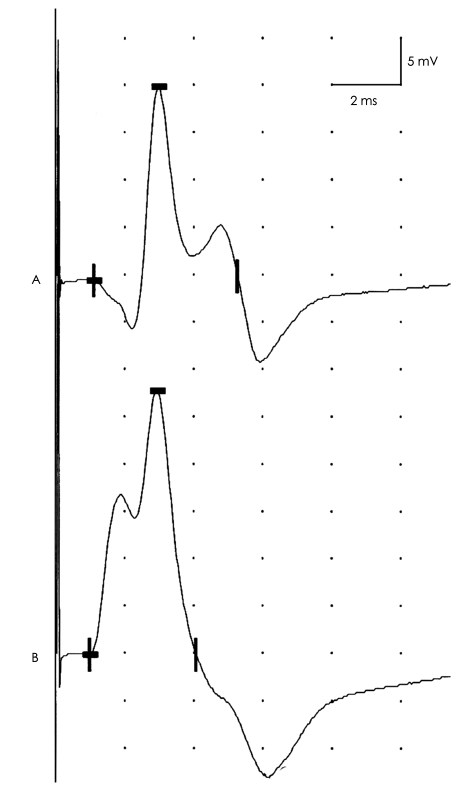
While recording electrodes are usually placed properly, inadequately secured electrodes can sometimes become dislodged, especially when motor NCSs are performed that involve vigorous muscle contractions.1 This might result in erroneous recording of various CMAP parameters (Fig. 9).
Fig. 9.
Effect of recording electrode position. CMAPs recorded from the adductor digiti minimi in response to ulnar nerve stimulation at the wrist. Attachment of the active electrode slightly away from the muscle's motor point results in an initial positive deflection and reduced CMAP amplitude (A). By relocating the active electrode to directly above the muscle's motor point, an initially negative biphasic CMAP is observed with increased amplitude (B). When a CMAP has an initial positive deflection, the active electrode should be relocated to the assumed motor point. CMAP: compound muscle action potential.
Limb position and anatomic nerve course
When attaching the surface electrodes to the patient's limb it is necessary to carefully consider the limb position and the anatomic course of the nerve under study. The lengths of limb nerves measured at the skin surface are reported to be 3-8 mm shorter than the actual length measured on cadaveric dissection.10 The most significant discrepancy between the length of nerves obtained by surface measurement and the actual length usually develops when measuring the course of a nerve across a joint. Measurement of the ulnar nerve, which is significantly affected by elbow position, is particularly prone to this type of measurement error.19 The ulnar nerve within the ulnar groove may become loose or follow a tortuous course during elbow extension, while elbow flexion stretches the nerve at the ulnar groove, so that surface measurement of the ulnar nerve across the elbow more closely reflects the true length of the ulnar nerve. It is therefore recommended that the elbow be flexed at 135° or 90° to minimize error during NCS of the ulnar nerve across the elbow section. However, this position may not be optimal because of the possibility of a hypermobile ulnar nerve at the elbow, as recently reemphasized by high-resolution ultrasonography.
Previous studies with normal healthy subjects revealed that the ulnar nerve is displaced in about 20-30% of limbs.20 In some cases, elbow flexion causes the ulnar nerve to be dislocated from the ulnar groove, across the medial epicondyle, to the volar side.21-23 A study comparing the surface-measured distance between stimulus points across the elbow (reflecting the length of the ulnar nerve) and its true length, as measured using ultrasonography, revealed a significant discrepancy that resulted in a large error in the calculated nerve conduction velocity between these stimulus points.19 Another study applied a short-segment NCS to a dislocated ulnar nerve at the elbow, which revealed the possibility of false-positive findings due to insufficient stimulation, and the authors recommended that short-segment NCSs at the elbow should be performed with the elbow flexed by less than 90°.24
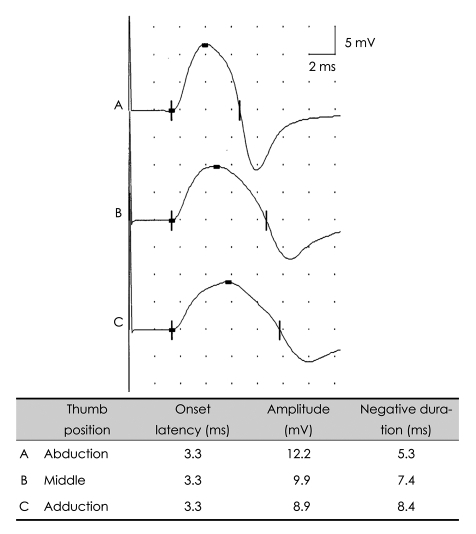
When the CMAP of the abductor pollicis brevis (APB) muscle was recorded by stimulating the median nerve, muscle shortening with relaxation resulted in a decreased CMAP duration and increased CMAP amplitude (Fig. 10).25 This finding implies that the hand position should be kept constant while examining the median motor nerves. Similarly, repetitive nerve stimulation studies are known to be affected by hand posture.26
Fig. 10.
Effect of limb muscle position. CMAPs recorded from abductor pollicis brevis with median nerve stimulation at the wrist. Alteration of muscle length as a result of different thumb positions affects CMAP parameters. The finger position should be kept constant during motor nerve conduction studies of the upper limbs in order to reduce the effect of muscle length on CMAP amplitude. CMAP: compound muscle action potential.
Stimulation
Stimulus artifact
During stimulation, the initial pulse of voltage from the stimulator creates a stimulus artifact - a far - field potential-that obscures the early part of any recorded waveforms.10 This stimulus artifact may make it difficult to interpret the waveforms when the stimulator and recording electrodes are relatively close.
Since stimulation with a higher current or longer duration produces a greater stimulus artifact, the applied current strength and duration should be just sufficient to elicit a supramaximal response. The stimulus artifact can be reduced by using a needle cathode instead of using a surface cathode. Furthermore, the impedance between the skin and all of the electrodes should be minimized by, for example, cleansing or rubbing the skin surface, shaving the skin, and/or using gel during electrode placement. Care should be taken to avoid using excessive amounts of gel or perspiration that could result in aberrant conduction pathways between the recording and reference electrodes, which would increase the stimulus artifact. The artifact can be reduced by using the same type of material for both E1 and E2 and by placing a ground electrode between E1 and the stimulator. In addition, anodal rotation about the cathode alters the distribution of isopotential lines at the two recording electrodes so that the amplified difference in potential between the electrodes can be markedly reduced.27 Differential amplification and common-mode rejection also reduces the artifact, especially when the stimulus artifacts at E1 and E2 are similar. A recent study proposed a hardware-based model of the stimulus artifact that can be used to estimate and then subtract the artifact.28
Stimulus lead
A strong stimulus intensity results in a large zone of depolarization, creating a so-called stimulus lead.1 In other words, a larger stimulus intensity will lengthen the segment of depolarized nerve beneath the cathode, resulting in a shorter latency. The connective tissue between the nerve and the stimulator contributes to the impedance, and this varies along the nerve. If the same current is applied at the nerve where the impedance is greater, a submaximal neural excitatory pulse might be produced, producing a reduced CMAP amplitude and prolonged latency. This mimics conduction block between the two stimulus sites, producing a false-positive finding or possibly accentuating a mild abnormality, especially in a short-segment NCS.
Inadvertent stimulation/recording
Due to the short distance between the ulnar and median nerves at the wrist, high stimulus current intensities might stimulate both of these nerves.1 For example, high-intensity stimulation might produce a small CMAP even after stimulating a median nerve with a complete lesion at the wrist. Such a CMAP should be suspected to have originated from ulnar nerve excitation through volume conduction.
Cathode/anode stability
The use of excessive gel could result in the stimulator slipping away from the nerve during stimulation.1 Care is especially important during repetitive nerve stimulation studies, since such slippage can result in false-positive findings.
Anodal stimulation
The anodal current theoretically hyperpolarizes the neural tissue beneath the anode, preventing action potential propagation past the anode site, which has been termed "anodal block".1 In 1993, Dreyer et al.29 showed that the CMAP and F-wave can be elicited by monopolar anodal stimulation, and the authors predicted that "anodal block" was unlikely to occur during routine electrodiagnostic studies. Moreover, the anode is known to generate action potentials,30,31 which may be caused by stimulating skin receptors and intradermal nerve endings, while the cathode directly activates underlying nerves.30,32 Therefore, the anode should be placed carefully so that it does not stimulate other nerves, possibly leading to false-negative findings. For example, during a blink-reflex study the anode may depolarize the contralateral supraorbital nerve, thereby producing a bilateral stimulation, which results in bilateral simultaneous R1 and R2 signals.1
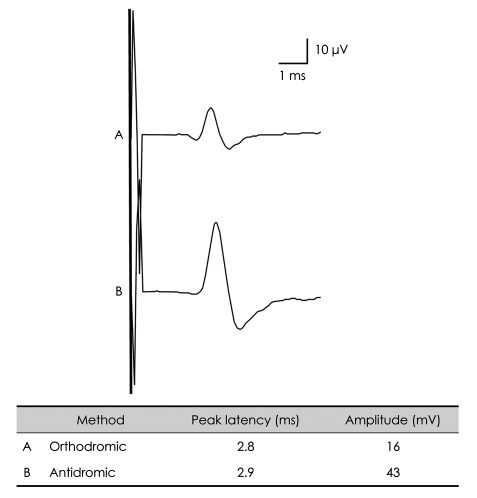
Pressure on the electrodes
The high pressure of the surface recording electrode on the skin decreases the distance between the electrode and the nerve and reduces the volume of tissue fluids between the skin and the nerve, thereby increasing the amplitude of the SNAP by decreasing the resistance between E1 and the nerve.8 Orthodromic SNAP amplitudes vary greatly while performing this method depending upon the extent of pressure on the nerve due to the presence of larger amounts of subcutaneous tissue between E1 and the nerve. This explains why orthodromic SNAP amplitudes are smaller than their antidromic counterparts (Fig. 11).1
Fig. 11.
Orthodromic versus antidromic recordings: median nerve SNAPs. The SNAP amplitude of the median nerve is smaller when using an orthodromic method (A) than when using an antidromic method (B). The interelectrode distances between the active electrode (E1) and the reference electrode (E2) were set to be the same (4 cm). SNAP: sensory nerve action potential.
H-reflex
The H-reflex is a monosynaptic reflex that involves the Ia afferents and alpha motoneurons.33 A stimulus pulse duration of 0.5-1.0 ms is recommended because longer current durations are believed to activate the large sensory fibers preferentially relative to the smaller motor fibers.
The amplitudes of both the H-reflex and M wave increase linearly with the stimulation intensity until the H-reflex reaches a maximum at certain stimulus intensities.34,35 As the stimulation intensity increases, more muscles are activated until the maximum M wave is reached, representing maximal muscle activation. According to the "size principle",36-38 smaller motoneurons, which are more excitable, will be recruited first when increasing the nerve stimulation to evoke the H-reflex. After reaching the maximum the H-reflex amplitude will progressively decline. Although the mechanism underlying this decline remains to be elucidated, it probably results from a combination of refractory alpha motoneurons and Renshaw inhibition of the motoneuron pool.33
With the H-reflex, the synaptic connection between Ia afferents and alpha motoneurons is known to be modulated by various spinal inhibitory interneuronal circuits.35 The H-reflex should thus be determined to be absent only after applying certain facilitation methods or maneuvers such as contraction of agonist muscles, the Jendrassik maneuver, posttetanic stimulation, and even ensure that the patient is focusing on a motor task.39,40
Interpretation
CMAP morphology
Obtained waveforms should be carefully inspected before an examiner accepts the results. Inappropriate location of the active electrode (which should be positioned directly over the motor point, the endplate zone) should be considered if a CMAP has an initial positive deflection.1 However, there are some conditions under which an initial negative deflection cannot be obtained regardless of the location of the active electrode, such as 1) the combination of carpal tunnel syndrome and a Martin-Gruber anastomosis (MGA), and 2) a distorted motor point caused by trauma and nerve injury to the muscle.
When a CMAP is recorded from the second lumbrical muscle following median nerve stimulation at the wrist for a comparison study in patients with suspicious carpal tunnel syndrome, a small negative potential - called the premotor potential - precedes the CMAP. A high amplifier sensitivity will help in identifying the premotor potential. The origin of this premotor potential has been controversial, but a recent study suggested that it originates from a median SNAP arising from antidromically activated digital sensory branches.41 Therefore, terminal latency of the CMAP from the second lumbrical muscle following median nerve stimulation should be considered as a point of initial negative deflection of a larger negative wave following the small premotor potential.
Amplitude variability
There is a physiologic decline in both the SNAP and CMAP amplitudes as the stimulation point moves further from the active electrode.1 A recent study42 investigated physiological changes of CMAPs obtained from stimulation at different sites over the full length of the ulnar motor nerve. The CMAP amplitude decreased linearly while its duration increased linearly with increasing conduction distance. The CMAP area decreased with the square of the conduction distance, but the decrease in area (up to 25% at the axilla) was smaller than the decrease in amplitude (up to 30% at the axilla). Recognizing this normal decrease in CMAP area and amplitude with longer distance, conduction block should be considered when the CMAP amplitude following proximal stimulation decreases by at least 50% compared to that following distal stimulation.
The variability in SNAP amplitude with stimulus location is considerably greater in antidromic sensory NCS than in motor studies;1 this difference is attributable to the greater temporal dispersion and larger difference between the fastest and slowest sensory neural fibers compared with motor nerves. An amplitude difference exceeding 50% is generally considered as pathologic, while a side-to-side amplitude difference of greater than 25% was considered abnormal in previous studies.43
Nonmodifiable physiologic factors
In addition to limb temperature, age, gender, and body mass index (BMI) should be considered as nonmodifiable factors affecting electrodiagnostic studies. The nerve conduction velocity reduces by approximately 1 m/s/decade of age between the ages of 20 and 80 years.1 Limb temperatures must be controlled with particular care in elderly persons, since the high prevalence of loss of muscle mass in this group may predispose them to cool limbs. Tong et al.44 reported a cohort study estimating how sensory NCS parameters change over time when subjects are measured at two time points, about 5 years apart. After controlling for confounding factors such as gender, age, height, and BMI, the amplitudes of the median and ulnar SNAPs decreased by about 2.3 and 1.75 µV over 5 years, respectively. Furthermore, the median and ulnar conduction velocities decreased by 1.1 and 0.71 m/s, respectively.
Gender-related differences have also been noted. Antidromic median, ulnar, and radial SNAPs are reported to have larger amplitudes and shorter distal latencies in women than in men.1 Although shorter latencies and faster velocities in women are unexplained, larger-amplitude antidromic digital SNAPs for women may be attributable to a smaller digit circumference producing a lower subcutaneous tissue-to-nerve tissue ratio. Negative findings in studies using the orthodromic SNAP technique45 also support this explanation.
A recent study46 applied multivariate analyses to systematically investigate the effects of age, gender, and BMI on SNAP amplitudes and nerve conduction velocities of both the upper and lower limbs. Age was negatively correlated with SNAP amplitudes for all of the nerves studied, but the extent of these effects varied in different nerves. For example, aging affected the median sensory nerve more than the radial sensory nerve. This might be explained by the superficial radial nerve not including the common entrapment site, whereas the median sensory nerve is entrapped within the carpal tunnel and is prone to repetitive injuries. BMI appears to significantly affect the amplitudes of only the upper-limb nerves, and not the lower-limb nerves, for which there is only a small amount of subcutaneous tissue between the stimulator and the nerve.
Height or limb length
While many studies have found that nerve conduction velocity is inversely correlated with limb length, only one study has controlled for temperature, and it yielded a negative result.1 There has since been general agreement that no correlation exists between upper-limb length and nerve conduction velocity.
Limitations of NCSs
Despite small-fiber neuropathy being a commonly encountered disorder, routine NCSs assess only large-fiber function.47 Therefore, some alternative methods are being used to study small fibers: sympathetic skin response,48 quantitative sudomotor axon reflex test,49 laser-evoked potential,50 cardiovagal and adrenegeric autonomic testing,51 and the cutaneous silent period.52,53
Electromyography
Clinical evaluation
Anamnesis and physical examination are essential before conducting EMG. Although there have been many attempts to increase the diagnostic value of anamnesis and physical examination in patients with lumbosacral radiculopathy,54-58 needle EMG provides additional diagnostic value in this disorder relative to clinical data and MRI.56 The usefulness of EMG in clinical practice is the ability to determine the pathophysiological mechanism underlying pain. Although needle EMG appears to have a rather low sensitivity (60%), it may reveal clinically relevant nerve dysfunction in patients where imaging findings are normal. Furthermore, needle EMG has excellent specificity for lumbosacral radiculopathy and plexopathy when appropriate diagnostic criteria are used.59
Equipment settings
Needle type
Two types of needle electrode, monopolar and concentric, are currently used for the routine examination of skeletal muscle.1 For the concentric needle electrode, the active and reference recording surfaces are in close proximity, facilitating common-mode rejection. However, the monopolar needle electrode has its reference electrode on the skin at some distance from the active recording site, thus diminishing the usefulness of common-mode rejection. Therefore, the concentric needle electrode recording tends to be associated with less noise than that of the monopolar needle electrode. In addition, concentric needle recordings produce motor unit action potentials (MUAPs) with smaller amplitudes because comparatively fewer muscle fibers are in close proximity to the active recording surface than for the monopolar needle electrode.
Filters
Filter cutoff frequencies for needle EMG studies are not as critical as those for NCSs.1 A low-frequency cutoff of 2-20 Hz and a high-frequency cutoff of 10000-20000 Hz are commonly used when performing either quantitative or qualitative MUAP analysis. However, the use of low-frequency filter with a cutoff approaching 20 Hz requires a relatively stable baseline, which is achieved by eliminating low-frequency noise. When quantitative EMG studies are performed, a much lower low-frequency filter cutoff is commonly chosen. Although the effect of filter cutoff frequency on MUAP parameters is less significant than on NCS, lowering the high-frequency cutoff to much below 10000 Hz or further raising the low-frequency cutoff above 20 Hz could distort MUAP parameters.
Muscle selection
Once the equipment is ready for the EMG examination, the neurophysiologist should select the muscles to examine on the basis of the clinical hypotheses being tested.60 The selected muscles should be those that cause the least discomfort for the patient.
The accuracy of needle placement in muscles depends upon muscle location, size, depth, and the surrounding anatomy. In general, superficial, larger muscles in the lower extremity are easier to sample than the smaller and deeply located muscles in the upper extremity. The accuracy of needle placement can be increased by selectively activating the target muscle and ensuring that the MUAP rise time is less than 500 ms. Needle-placement techniques for specific muscles in which it is usually difficult to accurately place the needle have been evaluated.61 One such muscle among the paraspinal muscle group is the multifidus. For a diagnosis of radiculopathy, it is important to confirm whether the paraspinal muscles - which are innervated directly from the spinal roots - are involved. The multifidus in particular - which is innervated by a single root via the medial branch of the dorsal ramus, while other paraspinal muscles are innervated by multilevel roots - is of special interest to electromyographers for evaluating patients with suspicious radiculopathy. Various techniques have recently been developed to increase the accuracy of needle placement in the multifidus.61-63 The authors of these studies highlighted the nonoptimal accuracy of previous techniques and suggested a modified technique involving lower needle angulation relative to the skin surface and closer insertion from the midline. Even if such techniques could improve the accuracy of needle placement in the multifidus, there are conditions under which the target spinal level must be identified precisely. The identification of spinal level relies mostly upon palpation to accurately locate the anatomic sites using surface landmarks. However, the accuracy of palpation is significantly affected by the examiner's experience, and the presence of spinal anomalies (e.g., such as spina bifida and L6 vertebra) and obesity will contribute to palpation error. Therefore, confirmation of the involved root level by needle EMG at the multifidus might not be recommended.
Spontaneous activity
Fibrillation potentials or positive sharp waves are typically found in patients with myopathies or neuropathies with denervation. However, these waveforms are also reported in normal subjects, especially in lumbosacral64,65 and cervical66 paraspinal muscles. However, there is a possibility of endplate spikes with an atypical appearance mimicking the configurations of fibrillation potentials and positive sharp waves.67,68 A slower rate of firing (7.4 Hz) and irregular discharges are distinguishing features of atypical endplate spikes from abnormal spontaneous potentials. In spite of this criticism, we have confirmed that denervation potentials can be present in normal individuals, even after scrutinizing the waveforms in order to specifically exclude endplate spikes.66 These spontaneous potentials in normal healthy persons are thought to be caused by asymptomatic disc herniation with compression or chemical irritation, or stretch and entrapment of the posterior primary ramus resulting from excessive movement.69 The most important characteristic of abnormal spontaneous activity that could differentiate atypical endplate potentials is regularity. However, the assessment of regularity also requires special care because patients with a pacemaker could present spontaneous activity with regular firing during lower-cervical or thoracic EMG studies. The regularity, frequency, and correlation with the peripheral pulse can help to differentiate this pacemaker artifact from abnormal spontaneous activities.70
It is widely believed that abnormal spontaneous activity after injury on needle EMG appears at specific times, which follow the rule of innervating nerve length-dependent involvement of muscle. Some authors have warned that symptom duration and spontaneous activities do not seem to be related to each other.71-74 Although those authors caution against interpreting electrodiagnostic findings based on symptom duration, the evolution of EMG findings could be correlated with the time after injury and the severity of axonal damage.70
Anomalous Innervation
CMAP morphology or amplitudes might appear to be abnormal even in the absence of technical errors. In these instances, anomalous innervation should always be considered.
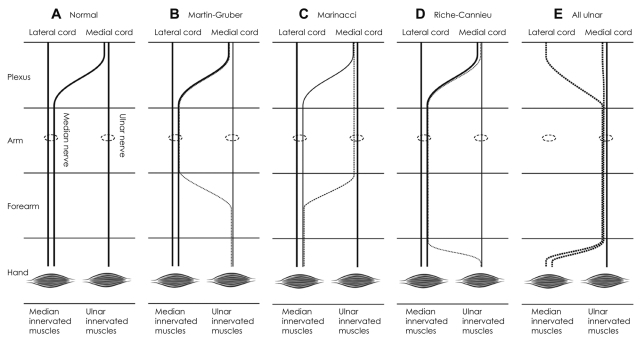
Martin-Gruber anastomosis
Normally, there is no connection between median nerve and ulnar nerve in the upper extremity (Fig. 12A). MGA refers to the innervation of the ulnar-innervated intrinsic hand muscles being supplied anomalously by fibers that travel with the median nerve in the arm and forearm (Fig. 12B).10 The incidence of this anastomosis varies depending upon the methodology used to evaluate it. Anatomical studies have revealed an incidence of 13.1-39.2%,75-78 while that determined by electrophysiological studies was 17-54%.79-81 In one study78 involving 68 adult Korean cadavers, the prevalence of MGA was 39.2%. The MGA was classified into four types, the most frequently occurring being type III (40%), in which an anastomosis exists between branches innervating the flexor digitorum profundus.
Fig. 12.
Simplified schemes of anomalous upper-limb innervations: examples of anomalous upper-limb innervations. Normally, there is no connection between median nerve and ulnar nerve (A). In Martin-Gruber anastomosis (B), those muscles that would normally be supplied by the ulnar nerve are supplied by median nerve fibers, which cross in the forearm. In Marinacci communication (C), those muscles that would normally be supplied by the median nerve are supplied by ulnar nerve fibers that cross in the forearm. In Riche-Cannieu anastomosis (D), those muscles that would normally be supplied by the ulnar nerve are supplied by median nerve fibers that cross in the hand. In "all ulnar hand" (E), the median nerve is absent and all of the muscles that would usually be supplied by the median nerve are innervated by the ulnar nerve. Dotted lines represent anomalous nerve fibers.
Electrophysiological evaluation of the MGA includes comparisons of the CMAP,82 the collision technique,83 and pharmacological blockade. However, these techniques can result in stimulus spread that results in the activation of other nerves.84 In order to avoid this problem, near-nerve stimulation may be used with almost right-to-the-tip Teflon-insulated unipolar needle electrodes.82,85
The common MGA often coexists with carpal tunnel syndrome, which is the most common entrapment neuropathy.86 In mild or moderately severe carpal tunnel syndrome, the MGA response superimposed on the median CMAP may result in an initial positive deflection as well as a faster conduction velocity. In more severe cases of carpal tunnel syndrome, the MGA response is separated from the median CMAP, producing an unrealistic negative conduction velocity.
MGA causing a decline in ulnar CMAP amplitude across the elbow segment could mimic a partial conduction block, which would suggest ulnar neuropathy at the elbow.87,88 Therefore, all patients with suspected ulnar neuropathy with a reduction in CMAP amplitude and area at the elbow segment must be evaluated for a proximal MGA before diagnosing partial conduction block, especially when there is no other evidence of ulnar neuropathy, such as a slower conduction velocity, abnormal SNAPs, and abnormal needle EMG findings. A recent study89 reinforces this suggestion with two examples of MGA combined with anomalous superficial radial innervation to the ulnar dorsum of the hand that resulted in the absence of a dorsal ulnar cutaneous response.
While MGA usually involves only motor fibers, a crossover of sensory fibers has also been detected by using a near-nerve technique.90
Marinacci communication
Marinacci communication occurs when ulnar fibers cross into the median nerve (Fig. 12C).80 A recent article reported that the prevalence of Marinacci communication is 4%.91 Due to the rarity of this communication, other physiologic, pathologic, or technical reasons should be considered before diagnosing an ulnar-to-median anomaly.
Riche-Cannieu anastomosis
Riche-Cannieu anastomosis is an anatomic communication between the recurrent branch of the median nerve and the deep branch of the ulnar nerve in the hand (Fig. 12D). It has been suggested recently that this anomalous innervation, which is present in approximately 77% of hands,92 has an autosomal dominant pattern of inheritance.93
All ulnar hand
"All ulnar hand" refers to an extremely rare innervation of an ulnar-to-median anastomosis combined with Marinacci communication and Riche-Cannieu anastomosis.94 This anomaly develops as a result of rerouting of axons from their cervical roots at the level of the brachial plexus (Fig. 12E). If the CMAP from the APB in response to median stimulation is completely without supportive clinical evidence of severe carpal tunnel syndrome (motor weakness and muscle atrophy), stimulation of the ulnar nerve while recording at the APB should be performed in order rule out this possibility.
Accessory deep peroneal nerve
The accessory deep peroneal nerve (ADPN) traverses posterior to the lateral malleolus and innervates the lateral portion of the extensor digitorum brevis (EDB) muscle.1 One cadaveric study detected an ADPN in all of the 24 legs studied (100%).95 In contrast, electrophysiological studies indicate the presence of the ADPN innervating the EDB in 19-28% of the general population.96-99 This anomaly has an autosomal dominant pattern of inheritance.99,100
ADPN should be suspected when the peroneal CMAP in response to stimulation at the fibular head is greater than that in response to supramaximal ankle stimulation.1 It should be remembered that an excessive current can induce a volume-conducted response, which stimulates tibial nerve, thus contracting intrinsic foot muscles other than the EDB and mimicking the presence of an ADPN. If the ADPN is combined with a deep peroneal neuropathy between the fibular head and ankle, collision techniques can be used to localize the pathology.101 The EDB is sometimes innervated only by the ADPN with a lesion of the deep peroneal nerve, which might cause preservation of toe extension in patients with a deep peroneal neuropathy.102
Tibial innervation of the EDB
Innervation of the EDB by the tibial nerve is rarely reported as a normal variant.103,104 However, care is necessary before diagnosing this anomalous innervation, since it can be mimicked by supramaximal stimulation and volume conduction causing contraction of the intrinsic foot muscles.105
Conclusions
Electrodiagnostic medicine is useful for characterizing and diagnosing neuromuscular disorders. However, there are numerous technical and physiologic parameters that can confound accurate conclusions. A thorough understanding of the potential pitfalls will enable the use of electrodiagnostic studies in conjunction with clinical examination to more accurately diagnose and treat patients with neuromuscular disorders.
Acknowledgements
This study was supported by a grant K1032101 from the Korea University, Republic of Korea.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Dumitru D, Zwartz MJ. Electrodiagnostic medicine pitfalls. In: Dumitru D, Amato AA, Zwarts M, editors. Electrodiagnostic Medicine. 2nd ed. Philadelphia: Hanley & Belfus; 2001. pp. 541–577. [Google Scholar]
- 2.Goldfarb AR, Saadeh PB, Sander HW. Effect of amplifier gain setting on distal motor latency in normal subjects and CTS patients. Clin Neurophysiol. 2005;116:1581–1584. doi: 10.1016/j.clinph.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 3.Dumitru D, Zwartz MJ. Instrumentation. In: Dumitru D, Amato AA, Zwarts M, editors. Electrodiagnostic Medicine. 2nd ed. Philadelphia: Hanley & Belfus; 2001. pp. 69–97. [Google Scholar]
- 4.Dumitru D, Walsh NE. Practical instrumentation and common sources of error. Am J Phys Med Rehabil. 1988;67:55–65. doi: 10.1097/00002060-198804000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Oh SJ. Basic components of electromyography instruments. In: Oh SJ, editor. Clinical Electromyography: Nerve Conduction Studies. 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2002. pp. 25–36. [Google Scholar]
- 6.van Dijk JG, Tjon-a-Tsien A, van der Kamp W. CMAP variability as a function of electrode site and size. Muscle Nerve. 1995;18:68–73. doi: 10.1002/mus.880180110. [DOI] [PubMed] [Google Scholar]
- 7.Lee EJ, Baek DH, Baek JY, Kim BJ, Choi J, Pak JJ, et al. PDMS- and silver-ball-based flexible multichannel surface electrode: fabrication and application in nerve conduction study on patients with diabetic polyneuropathy. IEEE Sens J. 2009;9:625–632. [Google Scholar]
- 8.Ven AA, Van Hees JG, Stappaerts KH. Effect of size and pressure of surface recording electrodes on amplitude of sensory nerve action potentials. Muscle Nerve. 2004;30:234–238. doi: 10.1002/mus.20071. [DOI] [PubMed] [Google Scholar]
- 9.Drenthen J, Blok JH, van Heel EB, Visser GH. Limb temperature and nerve conduction velocity during warming with hot water blankets. J Clin Neurophysiol. 2008;25:104–110. doi: 10.1097/WNP.0b013e31816a3b28. [DOI] [PubMed] [Google Scholar]
- 10.Dumitru D, Amato AA, Zwartz MJ. Nerve conduction studies. In: Dumitru D, Amato AA, Zwarts M, editors. Electrodiagnostic Medicine. 2nd ed. Philadelphia: Hanley & Belfus; 2001. pp. 159–223. [Google Scholar]
- 11.Eduardo E, Burke D. The optimal recording electrode configuration for compound sensory action potentials. J Neurol Neurosurg Psychiatry. 1988;51:684–687. doi: 10.1136/jnnp.51.5.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee HJ, DeLisa JA, Bach JR. Physiologic considerations in the determination of optimum interelectrode distance for the antidromic recording of compound sensory nerve action potentials. Commentary. Am J Phys Med Rehabil. 1993;72:99–100. doi: 10.1097/00002060-199304000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Oh SJ. Nerve conduction techniques. In: Oh SJ, editor. Clinical Electromyography: Nerve Conduction Studies. 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2002. pp. 37–53. [Google Scholar]
- 14.Varghese G, Rogoff JB. Influence of inter-electrode distance on antidromic sensory potentials. Electromyogr Clin Neurophysiol. 1983;23:297–301. [PubMed] [Google Scholar]
- 15.Evanoff V, Jr, Buschbacher RM. Optimal interelectrode distance in sensory and mixed compound nerve action potentials: 3- versus 4-centimeter bar electrodes. Arch Phys Med Rehabil. 2004;85:405–408. doi: 10.1016/s0003-9993(03)00617-8. [DOI] [PubMed] [Google Scholar]
- 16.Brashear A, Kincaid JC. The influence of the reference electrode on CMAP configuration: leg nerve observations and an alternative reference site. Muscle Nerve. 1996;19:63–67. doi: 10.1002/(SICI)1097-4598(199601)19:1<63::AID-MUS8>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 17.Kincaid JC, Brashear A, Markand ON. The influence of the reference electrode on CMAP configuration. Muscle Nerve. 1993;16:392–396. doi: 10.1002/mus.880160408. [DOI] [PubMed] [Google Scholar]
- 18.Nandedkar SD, Barkhaus PE. Contribution of reference electrode to the compound muscle action potential. Muscle Nerve. 2007;36:87–92. doi: 10.1002/mus.20798. [DOI] [PubMed] [Google Scholar]
- 19.Kim BJ, Date ES, Lee SH, Yoon JS, Hur SY, Kim SJ. Distance measure error induced by displacement of the ulnar nerve when the elbow is flexed. Arch Phys Med Rehabil. 2005;86:809–812. doi: 10.1016/j.apmr.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 20.Okamoto M, Abe M, Shirai H, Ueda N. Morphology and dynamics of the ulnar nerve in the cubital tunnel. Observation by ultrasonography. J Hand Surg Br. 2000;25:85–89. doi: 10.1054/jhsb.1999.0317. [DOI] [PubMed] [Google Scholar]
- 21.Childress HM. Recurrent ulnar-nerve dislocation at the elbow. Clin Orthop Relat Res. 1975:168–173. doi: 10.1097/00003086-197505000-00027. [DOI] [PubMed] [Google Scholar]
- 22.Grevsten S, Lindsjö U, Olerud S. Recurrent ulnar nerve dislocation at the elbow. Report of a non-traumatic case with ulnar entrapment neuropathy. Acta Orthop Scand. 1978;49:151–153. doi: 10.3109/17453677809005742. [DOI] [PubMed] [Google Scholar]
- 23.Jacobson JA, Jebson PJ, Jeffers AW, Fessell DP, Hayes CW. Ulnar nerve dislocation and snapping triceps syndrome: diagnosis with dynamic sonography--report of three cases. Radiology. 2001;220:601–605. doi: 10.1148/radiol.2202001723. [DOI] [PubMed] [Google Scholar]
- 24.Kim BJ, Koh SB, Park KW, Kim SJ, Yoon JS. Pearls & Oy-sters:false positives in short-segment nerve conduction studies due to ulnar nerve dislocation. Neurology. 2008;70:e9–e13. doi: 10.1212/01.wnl.0000297515.86197.2e. [DOI] [PubMed] [Google Scholar]
- 25.Kim BJ, Date ES, Park BK, Choi BY, Lee SH. Physiologic changes of compound muscle action potentials related to voluntary contraction and muscle length in carpal tunnel syndrome. J Electromyogr Kinesiol. 2005;15:275–281. doi: 10.1016/j.jelekin.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 26.Mori I, Hasegawa O. [Effect of posture and postural fixation in repetitive nerve stimulation test] No To Shinkei. 1999;51:867–870. [PubMed] [Google Scholar]
- 27.Kornfield MJ, Cerra J, Simons DG. Stimulus artifact reduction in nerve conduction. Arch Phys Med Rehabil. 1985;66:232–235. doi: 10.1016/0003-9993(85)90149-2. [DOI] [PubMed] [Google Scholar]
- 28.Tracey BH, Krishnamachari S. Automated removal of stimulus artifact in nerve conduction studies. Conf Proc IEEE Eng Med Biol Soc. 2006;1:6360–6363. doi: 10.1109/IEMBS.2006.260654. [DOI] [PubMed] [Google Scholar]
- 29.Dreyer SJ, Dumitru D, King JC. Anodal block V anodal stimulation. Fact or fiction. Am J Phys Med Rehabil. 1993;72:10–18. doi: 10.1097/00002060-199302000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Aprile I, Stålberg E, Tonali P, Padua L. Double peak sensory responses at submaximal stimulation. Clin Neurophysiol. 2003;114:256–262. doi: 10.1016/s1388-2457(02)00370-x. [DOI] [PubMed] [Google Scholar]
- 31.Yasunami T, Miyawaki Y, Kitano K, Okuno H. Shortening of distal motor latency in anode distal stimulation. Clin Neurophysiol. 2005;116:1355–1361. doi: 10.1016/j.clinph.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 32.Aprile I, Tonali P, Stalberg E, Di Stasio E, Caliandro P, Foschini M, et al. Double peak sensory responses: effects of capsaicin. Neurol Sci. 2007;28:264–269. doi: 10.1007/s10072-007-0833-x. [DOI] [PubMed] [Google Scholar]
- 33.Dumitru D, Zwartz MJ. Special nerve conduction techniques. In: Dumitru D, Amato AA, Zwarts M, editors. Electrodiagnostic Medicine. 2nd ed. Philadelphia: Hanley & Belfus; 2001. pp. 225–256. [Google Scholar]
- 34.Zehr EP. Considerations for use of the Hoffmann reflex in exercise studies. Eur J Appl Physiol. 2002;86:455–468. doi: 10.1007/s00421-002-0577-5. [DOI] [PubMed] [Google Scholar]
- 35.Knikou M. The H-reflex as a probe: pathways and pitfalls. J Neurosci Methods. 2008;171:1–12. doi: 10.1016/j.jneumeth.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 36.Somjen G, Carpenter DO, Henneman E. Responses of motoneurons of different sizes to graded stimulation of supraspinal centers of the brain. J Neurophysiol. 1965;28:958–965. doi: 10.1152/jn.1965.28.5.958. [DOI] [PubMed] [Google Scholar]
- 37.Henneman E, Somjen G, Carpenter DO. Excitability and inhibitability of motoneurons of different sizes. J Neurophysiol. 1965;28:599–620. doi: 10.1152/jn.1965.28.3.599. [DOI] [PubMed] [Google Scholar]
- 38.Awiszus F, Feistner H. The relationship between estimates of Ia-EPSP amplitude and conduction velocity in human soleus motoneurons. Exp Brain Res. 1993;95:365–370. doi: 10.1007/BF00229795. [DOI] [PubMed] [Google Scholar]
- 39.Hale BS, Raglin JS, Koceja DM. Effect of mental imagery of a motor task on the Hoffmann reflex. Behav Brain Res. 2003;142:81–87. doi: 10.1016/s0166-4328(02)00397-2. [DOI] [PubMed] [Google Scholar]
- 40.Kiers L, Fernando B, Tomkins D. Facilitatory effect of thinking about movement on magnetic motor-evoked potentials. Electroencephalogr Clin Neurophysiol. 1997;105:262–268. doi: 10.1016/s0921-884x(97)00027-1. [DOI] [PubMed] [Google Scholar]
- 41.Takahashi N, Takahashi O, Ogawa S, Takahashi M. What is the origin of the premotor potential recorded from the second lumbrical? Muscle Nerve. 2006;34:779–781. doi: 10.1002/mus.20606. [DOI] [PubMed] [Google Scholar]
- 42.Johnsen B, Fuglsang-Frederiksen A, de Carvalho M, Labarre-Vila A, Nix W, Schofield I. Amplitude, area and duration of the compound muscle action potential change in different ways over the length of the ulnar nerve. Clin Neurophysiol. 2006;117:2085–2092. doi: 10.1016/j.clinph.2006.05.033. [DOI] [PubMed] [Google Scholar]
- 43.Colachis SC, 3rd, Klejka JP, Shamir DY, Pease WS, Johnson EW. Amplitude of M responses. Side to side comparability. Am J Phys Med Rehabil. 1993;72:19–22. doi: 10.1097/00002060-199302000-00005. [DOI] [PubMed] [Google Scholar]
- 44.Tong HC, Werner RA, Franzblau A. Effect of aging on sensory nerve conduction study parameters. Muscle Nerve. 2004;29:716–720. doi: 10.1002/mus.20026. [DOI] [PubMed] [Google Scholar]
- 45.Shehab DK, Khuraibet AJ, Butinar D, Abraham MP, Jabre JF. Effect of gender on orthodromic sensory nerve action potential amplitude. Am J Phys Med Rehabil. 2001;80:718–720. doi: 10.1097/00002060-200110000-00002. [DOI] [PubMed] [Google Scholar]
- 46.Fujimaki Y, Kuwabara S, Sato Y, Isose S, Shibuya K, Sekiguchi Y, et al. The effects of age, gender, and body mass index on amplitude of sensory nerve action potentials: multivariate analyses. Clin Neurophysiol. 2009;120:1683–1686. doi: 10.1016/j.clinph.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 47.Lacomis D. Small-fiber neuropathy. Muscle Nerve. 2002;26:173–188. doi: 10.1002/mus.10181. [DOI] [PubMed] [Google Scholar]
- 48.Vetrugno R, Liguori R, Cortelli P, Montagna P. Sympathetic skin response: basic mechanisms and clinical applications. Clin Auton Res. 2003;13:256–270. doi: 10.1007/s10286-003-0107-5. [DOI] [PubMed] [Google Scholar]
- 49.Tobin K, Giuliani MJ, Lacomis D. Comparison of different modalities for detection of small fiber neuropathy. Clin Neurophysiol. 1999;110:1909–1912. doi: 10.1016/s1388-2457(99)00164-9. [DOI] [PubMed] [Google Scholar]
- 50.Rossi P, Serrao M, Amabile G, Parisi L, Pierelli F, Pozzessere G. A simple method for estimating conduction velocity of the spinothalamic tract in healthy humans. Clin Neurophysiol. 2000;111:1907–1915. doi: 10.1016/s1388-2457(00)00442-9. [DOI] [PubMed] [Google Scholar]
- 51.Low PA. Testing the autonomic nervous system. Semin Neurol. 2003;23:407–421. doi: 10.1055/s-2004-817725. [DOI] [PubMed] [Google Scholar]
- 52.Floeter MK. Cutaneous silent periods. Muscle Nerve. 2003;28:391–401. doi: 10.1002/mus.10447. [DOI] [PubMed] [Google Scholar]
- 53.Kim BJ, Kim NH, Kim SG, Roh H, Park HR, Park MH, et al. Utility of the cutaneous silent period in patients with diabetes mellitus. J Neurol Sci. 2010;293:1–5. doi: 10.1016/j.jns.2010.03.032. [DOI] [PubMed] [Google Scholar]
- 54.Vroomen PC, de Krom MC, Knottnerus JA. Diagnostic value of history and physical examination in patients suspected of sciatica due to disc herniation: a systematic review. J Neurol. 1999;246:899–906. doi: 10.1007/s004150050480. [DOI] [PubMed] [Google Scholar]
- 55.Vroomen PC, de Krom MC, Knottnerus JA. Consistency of history taking and physical examination in patients with suspected lumbar nerve root involvement. Spine (Phila Pa 1976) 2000;25:91–96. doi: 10.1097/00007632-200001010-00016. discussion 97. [DOI] [PubMed] [Google Scholar]
- 56.Coster S, de Bruijn SF, Tavy DL. Diagnostic value of history, physical examination and needle electromyography in diagnosing lumbosacral radiculopathy. Neurol. 2010;257:332–337. doi: 10.1007/s00415-009-5316-y. [DOI] [PubMed] [Google Scholar]
- 57.Suri P, Rainville J, Katz JN, Jouve C, Hartigan C, Limke J, et al. The accuracy of the physical examination for the diagnosis of midlumbar and low lumbar nerve root impingement. Spine (Phila Pa 1976) 2011;36:63–73. doi: 10.1097/BRS.0b013e3181c953cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van der Windt DA, Simons E, Riphagen II, Ammendolia C, Verhagen AP, Laslett M, et al. Physical examination for lumbar radiculopathy due to disc herniation in patients with low-back pain. Cochrane Database Syst Rev. 2010:CD007431. doi: 10.1002/14651858.CD007431.pub2. [DOI] [PubMed] [Google Scholar]
- 59.Tong HC, Haig AJ, Yamakawa KS, Miner JA. Specificity of needle electromyography for lumbar radiculopathy and plexopathy in 55- to 79-year-old asymptomatic subjects. Am J Phys Med Rehabil. 2006;85:908–912. doi: 10.1097/01.phm.0000242627.81326.6c. quiz 913-915, 934. [DOI] [PubMed] [Google Scholar]
- 60.Daube JR, Rubin DI. Needle electromyography. Muscle Nerve. 2009;39:244–270. doi: 10.1002/mus.21180. [DOI] [PubMed] [Google Scholar]
- 61.Kim BJ, Date ES, Derby R, Lee SH, Seo KS, Oh KJ, et al. Electromyographic technique for lumbar multifidus examination: comparison of previous techniques used to localize the multifidus. Arch Phys Med Rehabil. 2005;86:1325–1329. doi: 10.1016/j.apmr.2004.12.027. [DOI] [PubMed] [Google Scholar]
- 62.Haig AJ, Moffroid M, Henry S, Haugh L, Pope M. A technique for needle localization in paraspinal muscles with cadaveric confirmation. Muscle Nerve. 1991;14:521–526. doi: 10.1002/mus.880140606. [DOI] [PubMed] [Google Scholar]
- 63.Stein J, Baker E, Pine ZM. Medial paraspinal muscle electromyography: techniques of examination. Arch Phys Med Rehabil. 1993;74:497–500. doi: 10.1016/0003-9993(93)90113-o. [DOI] [PubMed] [Google Scholar]
- 64.Nardin RA, Raynor EM, Rutkove SB. Fibrillations in lumbosacral paraspinal muscles of normal subjects. Muscle Nerve. 1998;21:1347–1349. doi: 10.1002/(sici)1097-4598(199810)21:10<1347::aid-mus20>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 65.Date ES, Mar EY, Bugola MR, Teraoka JK. The prevalence of lumbar paraspinal spontaneous activity in asymptomatic subjects. Muscle Nerve. 1996;19:350–354. doi: 10.1002/(SICI)1097-4598(199603)19:3<350::AID-MUS11>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 66.Date ES, Kim BJ, Yoon JS, Park BK. Cervical paraspinal spontaneous activity in asymptomatic subjects. Muscle Nerve. 2006;34:361–364. doi: 10.1002/mus.20557. [DOI] [PubMed] [Google Scholar]
- 67.Dumitru D, Diaz CA, King JC. Prevalence of denervation in paraspinal and foot intrinsic musculature. Am J Phys Med Rehabil. 2001;80:482–490. doi: 10.1097/00002060-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 68.Dumitru D. Physiologic basis of potentials recorded in electromyography. Muscle Nerve. 2000;23:1667–1685. doi: 10.1002/1097-4598(200011)23:11<1667::aid-mus2>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 69.Haig AJ. Paraspinal denervation and the spinal degenerative cascade. Spine J. 2002;2:372–380. doi: 10.1016/s1529-9430(02)00201-2. [DOI] [PubMed] [Google Scholar]
- 70.Date ES, Kim BJ. Cervical and thoracic radiculopathies. In: Kimura J, editor. Peripheral Nerve Diseases: Handbook of Clinical Neurophysiology. 1st ed. Vol 7. New York: Elsevier; 2006. pp. 601–612. [Google Scholar]
- 71.Dillingham TR, Pezzin LE, Lauder TD. Cervical paraspinal muscle abnormalities and symptom duration: a multivariate analysis. Muscle Nerve. 1998;21:640–642. doi: 10.1002/(sici)1097-4598(199805)21:5<640::aid-mus11>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 72.Pezzin LE, Dillingham TR, Lauder TD, Andary M, Kumar S, Stephens RR, et al. Cervical radiculopathies: relationship between symptom duration and spontaneous EMG activity. Muscle Nerve. 1999;22:1412–1418. doi: 10.1002/(sici)1097-4598(199910)22:10<1412::aid-mus11>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 73.Dillingham TR, Pezzin LE, Lauder TD. Relationship between muscle abnormalities and symptom duration in lumbosacral radiculopathies. Am J Phys Med Rehabil. 1998;77:103–107. [PubMed] [Google Scholar]
- 74.Dillingham TR, Pezzin LE, Lauder TD, Andary M, Kumar S, Stephens RT, et al. Symptom duration and spontaneous activity in lumbosacral radiculopathy. Am J Phys Med Rehabil. 2000;79:124–132. doi: 10.1097/00002060-200003000-00003. [DOI] [PubMed] [Google Scholar]
- 75.Srinivasan R, Rhodes J. The median-ulnar anastomosis (Martin-Gruber) in normal and congenitally abnormal fetuses. Arch Neurol. 1981;38:418–419. doi: 10.1001/archneur.1981.00510070052007. [DOI] [PubMed] [Google Scholar]
- 76.Rodriguez-Niedenführ M, Vazquez T, Parkin I, Logan B, Sañudo JR. Martin-Gruber anastomosis revisited. Clin Anat. 2002;15:129–134. doi: 10.1002/ca.1107. [DOI] [PubMed] [Google Scholar]
- 77.Rodriguez-Niedenführ M, Vazquez T, Ferreira B, Parkin I, Nearn L, Sañudo JR. Intramuscular Martin-Gruber anastomosis. Clin Anat. 2002;15:135–138. doi: 10.1002/ca.1108. [DOI] [PubMed] [Google Scholar]
- 78.Lee KS, Oh CS, Chung IH, Sunwoo IN. An anatomic study of the Martin-Gruber anastomosis: electrodiagnostic implications. Muscle Nerve. 2005;31:95–97. doi: 10.1002/mus.20141. [DOI] [PubMed] [Google Scholar]
- 79.Leibovic SJ, Hastings H., 2nd Martin-Gruber revisited. J Hand Surg Am. 1992;17:47–53. doi: 10.1016/0363-5023(92)90112-3. [DOI] [PubMed] [Google Scholar]
- 80.Amoiridis G, Vlachonikolis IG. Verification of the median-to-ulnar and ulnar-to-median nerve motor fiber anastomosis in the forearm: an electrophysiological study. Clin Neurophysiol. 2003;114:94–98. doi: 10.1016/s1388-2457(02)00328-0. [DOI] [PubMed] [Google Scholar]
- 81.Lee SA, Kim KK, Lee MC, Sunwoo IN, Kim KW. The electrodiagnostic findings in Martin-Gruber anastomosis. J Korean Neurol Assoc. 1994;12:87–91. [Google Scholar]
- 82.Amoiridis G. Median--ulnar nerve communications and anomalous innervation of the intrinsic hand muscles: an electrophysiological study. Muscle Nerve. 1992;15:576–579. doi: 10.1002/mus.880150507. [DOI] [PubMed] [Google Scholar]
- 83.Kimura J. Collision technique. Physiologic block of nerve impulses in studies of motor nerve conduction velocity. Neurology. 1976;26:680–682. doi: 10.1212/wnl.26.7.680. [DOI] [PubMed] [Google Scholar]
- 84.Amoiridis G, Schöls L, Przuntek H, Wöhrle J. Collision technique in Martin-Gruber anastomosis. Muscle Nerve. 1998;21:1354–1356. doi: 10.1002/(sici)1097-4598(199810)21:10<1354::aid-mus22>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 85.Amoiridis G. Fact and fallacy in electrophysiological studies of anomalous innervation patterns of the intrinsic hand muscles. Muscle Nerve. 1994;17:245–247. doi: 10.1002/mus.880170217. [DOI] [PubMed] [Google Scholar]
- 86.Rubin DI, Dimberg EL. Martin-Gruber anastomosis and carpal tunnel syndrome: [corrected] morphologic clues to identification. Muscle Nerve. 2010;42:457–458. doi: 10.1002/mus.21751. [DOI] [PubMed] [Google Scholar]
- 87.Marras C, Midroni G. Proximal Martin-Gruber anastomosis mimicking ulnar neuropathy at the elbow. Muscle Nerve. 1999;22:1132–1135. doi: 10.1002/(sici)1097-4598(199908)22:8<1132::aid-mus20>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 88.Whitaker CH, Felice KJ. Apparent conduction block in patients with ulnar neuropathy at the elbow and proximal Martin-Gruber anastomosis. Muscle Nerve. 2004;30:808–811. doi: 10.1002/mus.20128. [DOI] [PubMed] [Google Scholar]
- 89.Leis AA, Stetkarova I, Wells KJ. Martin-Gruber anastomosis with anomalous superficial radial innervation to ulnar dorsum of hand: a pitfall when common variants coexist. Muscle Nerve. 2010;41:313–317. doi: 10.1002/mus.21510. [DOI] [PubMed] [Google Scholar]
- 90.Simonetti S. Electrophysiological study of forearm sensory fiber crossover in Martin-Gruber anastomosis. Muscle Nerve. 2001;24:380–386. doi: 10.1002/1097-4598(200103)24:3<380::aid-mus1009>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 91.Meenakshi-Sundaram S, Sundar B, Arunkumar MJ. Marinacci communication: an electrophysiological study. Clin Neurophysiol. 2003;114:2334–2337. doi: 10.1016/s1388-2457(03)00260-8. [DOI] [PubMed] [Google Scholar]
- 92.Harness D, Sekeles E. The double anastomotic innervation of thenar muscles. J Anat. 1971;109(Pt 3):461–466. [PMC free article] [PubMed] [Google Scholar]
- 93.Boland RA, Krishnan AV, Kiernan MC. Riche-Cannieu anastomosis as an inherited trait. Clin Neurophysiol. 2007;118:770–775. doi: 10.1016/j.clinph.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 94.Kim BJ, Date ES, Lee SH, Lau EW, Park MK. Unilateral all ulnar hand including sensory without forearm communication. Am J Phys Med Rehabil. 2004;83:569–573. doi: 10.1097/01.phm.0000130034.76547.4f. [DOI] [PubMed] [Google Scholar]
- 95.Kudoh H, Sakai T, Horiguchi M. The consistent presence of the human accessory deep peroneal nerve. J Anat. 1999;194(Pt 1):101–108. doi: 10.1046/j.1469-7580.1999.19410101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lambert EH. The accessory deep peroneal nerve. A common variation in innervation of extensor digitorum brevis. Neurology. 1969;19:1169–1176. doi: 10.1212/wnl.19.12.1169. [DOI] [PubMed] [Google Scholar]
- 97.Murad H, Neal P, Katirji B. Total innervation of the extensor digitorum brevis by the accessory deep peroneal nerve. Eur J Neurol. 1999;6:371–373. doi: 10.1046/j.1468-1331.1999.630371.x. [DOI] [PubMed] [Google Scholar]
- 98.Neundörfer B, Seiberth R. The accessory deep peroneal nerve. J Neurol. 1975;209:125–129. doi: 10.1007/BF00314605. [DOI] [PubMed] [Google Scholar]
- 99.Owsiak S, Kostera-Pruszczyk A, Rowińska-Marcińska K. Accessory deep peroneal nerve - a clinically significant anomaly? Neurol Neurochir Pol. 2008;42:112–115. [PubMed] [Google Scholar]
- 100.Crutchfield CA, Gutmann L. Hereditary aspects of accessory deep peroneal nerve. J Neurol Neurosurg Psychiatry. 1973;36:989–990. doi: 10.1136/jnnp.36.6.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sander HW, Quinto C, Chokroverty S. Accessory deep peroneal neuropathy: collision technique diagnosis. Muscle Nerve. 1998;21:121–123. doi: 10.1002/(sici)1097-4598(199801)21:1<121::aid-mus17>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 102.Kayal R, Katirji B. Atypical deep peroneal neuropathy in the setting of an accessory deep peroneal nerve. Muscle Nerve. 2009;40:313–315. doi: 10.1002/mus.21324. [DOI] [PubMed] [Google Scholar]
- 103.Linden D, Berlit P. The intrinsic foot muscles are purely innervated by the tibial nerve ("all tibial foot")--an unusual innervation anomaly. Muscle Nerve. 1994;17:560–561. [PubMed] [Google Scholar]
- 104.Lee SY, Yoon SR, Choi IS, Lee SG, Rowe SM. Extensor digitorum brevis innervated by the tibial nerve (all tibial foot): a case report. J Korean Acad Rehabil Med. 2000;24:1223–1228. [Google Scholar]
- 105.Amoiridis G, Schöls L, Meves S, Przuntek H. Fact and fallacy in clinical and electrophysiological studies of anomalous innervation of the intrinsic foot muscles. Muscle Nerve. 1996;19:1227–1229. doi: 10.1002/mus.880190905. [DOI] [PubMed] [Google Scholar]