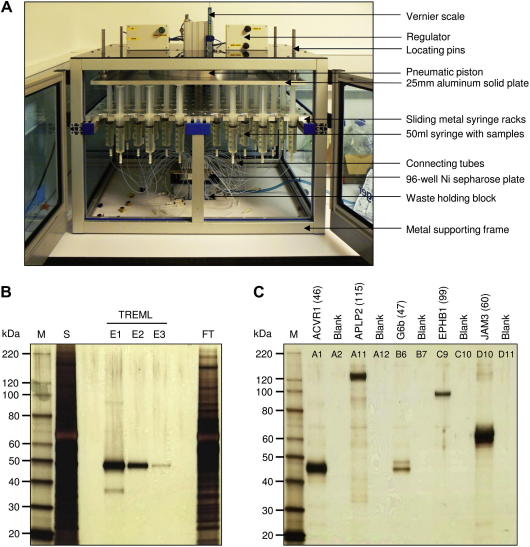
Fig.2.
A protein purification system for the parallel purification of 96 large tissue culture volumes. (A) The loading press can purify up to 96 50- to 100-ml samples of His-tagged proteins in parallel. It consists of a pneumatic piston attached to an aluminum plate that is used to drive tissue culture supernatants loaded in 50-ml disposable syringes through tubes that are connected to a holding block containing the 96-well Ni2+–NTA resin filter plate. (B) Proteins are purified to greater than 90% purity using the custom loading apparatus. A typical human cell surface receptor protein (TREML) was cloned into the BLH vector and expressed, and 50 ml of spent supernatant was loaded onto a single well of a 96-well microtiter plate containing Ni2+–NTA resin. Three serial 200-μl elutions were performed (E1–E3), and 20 μl of a 1:4000 dilution was loaded and resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) under reducing conditions and detected using silver staining. S = 18 μl spent supernatant; FT = 18 μl flow-through; M = markers. (C) No cross-well contamination is detected using the protein purification system. A purification experiment was set up with 48 transfection supernatants and intentionally included blank wells containing tissue culture medium alone to test for cross-well contamination. Eluates from the plate (including neighboring blank wells) were diluted 1:4000, and 20 μl was resolved by SDS–PAGE under reducing conditions and detected by silver staining. The gel shows representative purified bait proteins at the expected mass together with the eluate from neighboring blank wells showing no cross-well contamination.