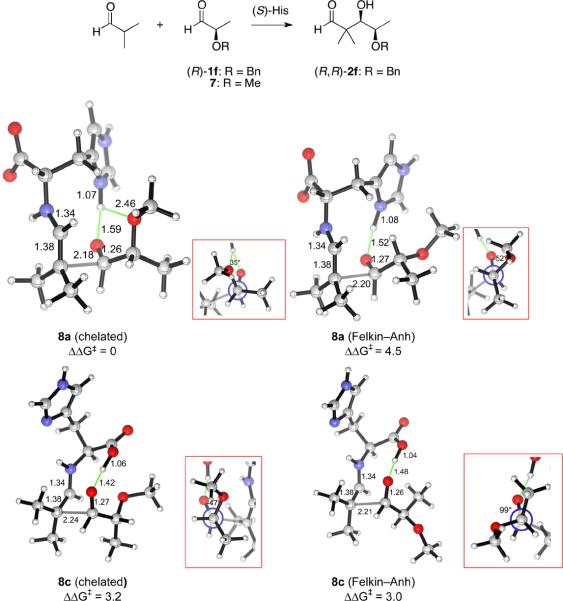
Figure 9.
Transition structures for the aldolization reaction of the histidine-enamine of isobutyraldehyde and 7, in which a hydrogen bond is donated by the imidazolium group (8a) or the carboxylic acid (8c). Two rotamers of the C–Cα bond for shown for 8a and 8c. 8a leads to the experimentally observed (R,R)-diastereomer, while 8c gives rise to the (S,R)-isomer.