Table 2.
Relative Free Energies (in kcal/mol) of Transition Structures with Imidazolium or Carboxylic Acid Group as Hydrogen Bond Donor
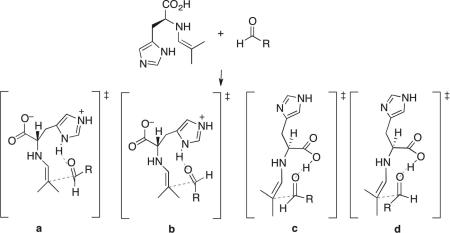 | |||||||
|---|---|---|---|---|---|---|---|
| Entry | R | ΔΔG‡ (a) | ΔΔG‡ (b) | ΔΔG‡ (c) | ΔΔG‡ (d) | ee computed | ee found |
| 1 | CH2Cl (1a) | 0 | 2.5 | 0.8 | 2.8 | 75% (R) | 90% (R) |
| 2 | CH(OMe)2 (1b) | 0 | 3.9 | 0.6 | 3.4 | 47% (R) | 84% (R) |
| 3 | CO2Et a (1c) | 0 | – a | 1.3 | – a | 80% (R) | 77% (R) |
| 4 | CH2SBn b (1d) | 0 | 1.7 | 0.6 | 1.0 | 47% (R) | 71% (R) |
| 5 | CH2i-Pr (1e) | 0 | 2.0 | 0.7 | 1.3 | 54% (S) | 56% (S) |
Methyl glyoxylate (R = CO2Me) was used as a model reactant. Transition structures of types b and c could not be located.
2-(Methylthio)acetaldehyde (R = CH2SMe) was used as a model reactant.
