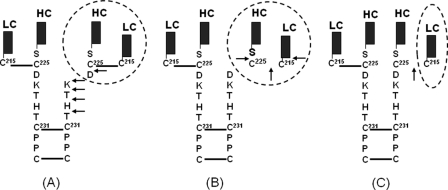
FIGURE 1.
Schematic illustration of the •OH radical induced hinge degradation in a human IgG1. The •OH radicals induce degradations of an IgG1 hinge to generate different products under different conditions. Under high oxygen tension, hinge cleavage releases Fab fragment and a partial IgG1 that is missing the Fab, with complementary ladders of C- and N-terminal hinge residues (Asp226, Lys227, Thr228, His229, and Thr230) of the upper hinge (panel A) (9); whereas under low oxygen tension, breakage of the heavy-light chain linkage leads to the peptide bond cleavage that releases light chain (LC) and Fab portion of the heavy chain (HC) (panel B), or the breakage of the heavy chain (HC) and light chain (LC) linkage without any cleavage of peptide bond (panel C) (11). The hinge degradation is initiated by radical formation at Cys231 resulting from breakage of the first hinge disulfide bond between two heavy chains by the •OH attacks, and followed by radical reactions via ET and localization onto the upper hinge or the inter-chain disulfide bond. The degraded products (circled) varies depending on the reaction conditions, as described previously (9–11).