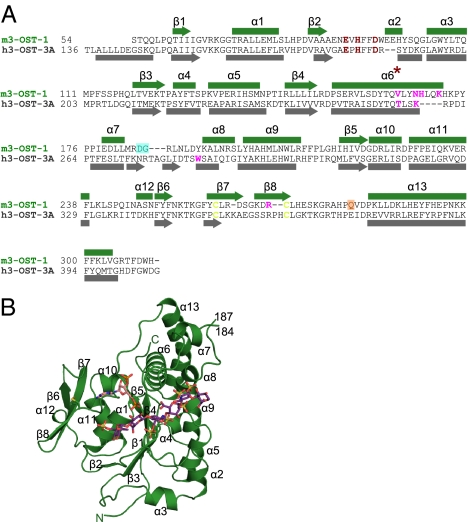
Fig. 1.
Sequence and structure of 3-O-sulfotransferase isoforms. (A) Structure-based sequence alignment of 3-OST-1 and 3-OST-3. Secondary structural elements are shown, numbered, and labeled, with α-helices as rectangles and β-sheets as arrows. Disordered residues Asp185–Gly186 (from molecules A and B) are shadowed by a cyan box. Residues disordered only in molecule B are shadowed by an orange box. Catalytic residues are colored in red, disulfides in yellow, and residues mutated in this study in magenta. (B) Ribbon diagram of m3-OST-1 with PAP (pink) and bound heptasaccharide substrate (purple). The substrate bound in molecule A is the most well ordered and will therefore be used in all figures. All secondary structural elements are numbered as in A. Disulfide bond between Cys260 and Cys269 is drawn in yellow. Structural figure was generated using PyMOL (18).