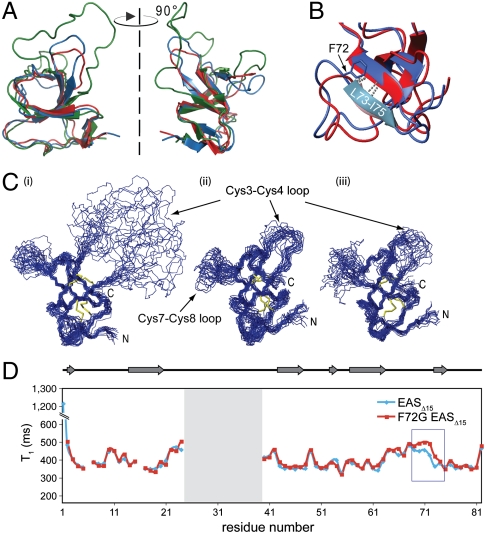
Fig. 4.
The F72G mutation does not disrupt the hydrophobin fold but has local effects on the backbone dynamics around the site of the mutation. (A) Overlay of the structures of WT EAS (green), EASΔ15 (blue), and F72G EASΔ15 (red) indicate that the β-barrel core structures are very similar. In WT EAS the Cys3–Cys4 loop is extended and highly mobile. (B) There are minor differences in the structures around the site of the mutation but the L73–I75 βstrand remains hydrogen bonded to the core of the F72G protein. (C) A comparison of the 20 lowest-energy structures calculated for (i) WT EAS, (ii) EASΔ15, and (iii) F72G EASΔ15 shows that the mutant protein adopts a fold similar to that of the parent structures. In all three structures, two disordered regions are observed, namely the segments between Cys3–Cys4 and Cys7–Cys8. (D) 15N relaxation experiments carried out on the EASΔ15-F72G mutant protein demonstrate a small but significant increase in the T1 relaxation time constant for residues around the site of mutation (boxed in blue), indicating an increase in flexibility as a result of the glycine substitution.