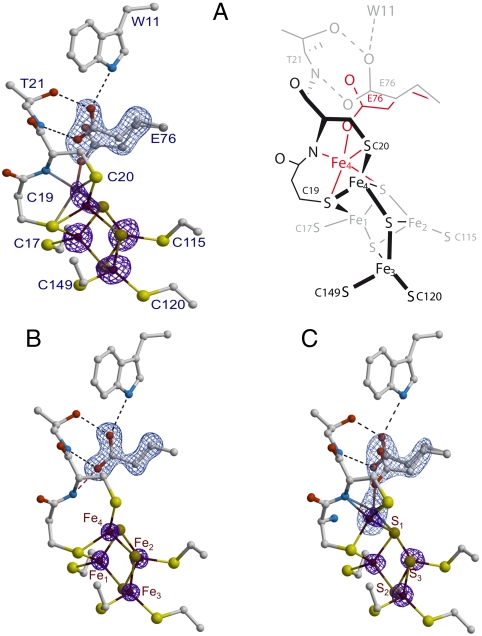
Fig. 2.
(A) As-isolated EcHyd-1 proximal cluster structure. Iron positions are depicted with their corresponding anomalous difference (Δanom) electron density peaks (Left) contoured at the 5σ level (purple). The omit map for Glu76 is depicted in light blue contoured at 8σ (the same color codes apply to (B) and (C). A schematic representation of the two conformations for Fe4 and Glu76 (black/gray and red) is shown on the right side of the figure. (B) H2-reduced cluster structure. The positions of Glu76 and Fe4 are as in O2-sensitive [NiFe]-hydrogenases. The peaks for the iron ions and the omit map are contoured at the 10 and 20σ levels, respectively. (C) The 4-OH-1,4-naphthoquinone/ferricyanide-oxidized structure. Glu76 has the alternative positions already observed in the as-isolated structure, whereas Fe4 is modeled with two nearby positions bound to S1, the Sγ and amide N from Cys20 and to either the Sγ of Cys19 or one Oϵ from Glu76. In both cases the iron coordination forms a distorted tetrahedron. The iron peaks and the omit map are contoured at the 6 and 9σ levels, respectively. Hydrogens have not been included in these figures.