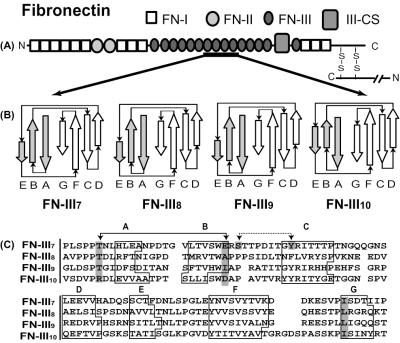
Figure 1.
Structure of the human FN monomer. (A) Modular structure of FN protein emphasizing folding motifs, FN-I, FN-II, and FN-III. (B) Secondary structures of selected FN-III modules with accurate representations of relative β-strand lengths with respect to each other. The upper sheets are shaded gray while lower sheets are white. (C) Sequence alignment of the four FN-III modules. FN-III7, FN-III8, FN-III9, and FN-III10 have 93, 91, 91, and 94 aa, respectively. Key residues discussed within the text are highlighted in dark gray, while residues forming key hydrogen bonds are connected by arrows.