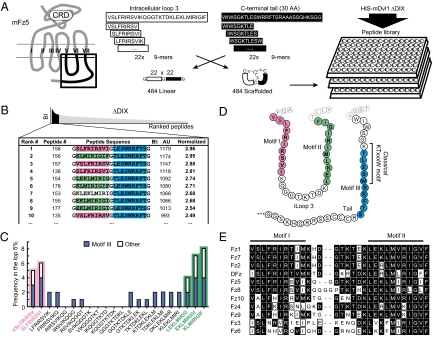
Fig. 1.
Identification of a discontinuous Dvl1 binding site in Fz5. (A) (Left) Schematic depiction of the topology of Fz proteins. iLoop3 and the C-terminal tail are boxed. (Right) Design of the Fz5 iLoop3-tail peptide library, based on 30 amino acids from mouse Fz5 iLoop3 (white) and 30 membrane-proximal amino acids from the Fz5 tail (black). Linear 9-mer peptides (22 each) were combined in cis, flanked and separated by Gly or Cys residues. CLIPS technology was used on Cys-based combinations, yielding bicyclical peptides; Gly-linked peptides were left linear. The combinatory peptides were immobilized in 455-well plates. Binding of recombinant His-tagged Dvl1-ΔDIX (G86–M695) was determined by an ELISA-based assay. (B) Binding signals were ranked by binding intensity (BI), and the 10 best binding peptides are depicted with their absolute score and normalized BI (relative to the screen average). AU, Arbitrary Units. (C) Frequency analysis of the top 5% (48 best binding peptides) reveals a strong preference for binding two motifs in iLoop3 (motifs I and II; pink and green) and one in the C terminus (motif III; blue). Motif III overlaps with the classic PDZ binding motif. Depicted are the frequencies of iLoop3 peptides in the top 5%, whether combined to motif III (blue) or not (white; other). (D) Diagram of the three Dvl1 binding motifs in the mFz5 iLoop3 and C-terminal tail. (E) Alignment of regions encompassing motifs I and II of mouse Fz family members and Drosophila Fz (DFz). Motifs I and II are highly conserved among most Fzs.