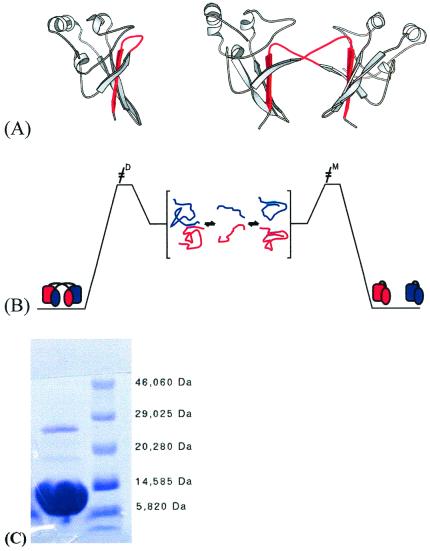
Figure 1.
Schematic representation of the structures of monomer and dimer forms of suc1 and model of domain swapping in suc1. (A) Monomer and dimer forms of suc1 coexist in solution in the absence of higher-order oligomers and aggregate over a wide range of conditions. The monomer form comprises a four-stranded β-sheet capped at one end by three short α-helices (26). In the dimer form, however, β-strand 4 (shown in red) originates from the other monomer in the dimer pair (27). There is no significant change in the fold except in the region that mediates the process, namely the hinge loop between β-strands 3 and 4. This loop (also colored red) adopts an extended conformation allowing β-strand 4 to exchange in the dimer, while it folds back on itself to form a β-hairpin in the monomer. The frequent occurrence of prolines in the hinge loops of domain-swapping proteins has been reported before (14). The β-hinge motif HxPEPH (where x is any residue) is conserved in the cks family. (B) Interconversion between monomer and dimer occurs via the denatured state. The residual interactions between β2 and the exchanging strand β4 that are retained in the denatured state are highlighted. (C) SDS/PAGE of wild-type suc1 at 0.6 mM protein concentration, showing two bands corresponding approximately to the molecular weights of monomer (13.3 kDa) and dimer (26.6 kDa). The protein sample was incubated in 2.5% SDS/6% β-mercaptoethanol at 95°C for 5 min before loading, and the gels were run by using the Phastgel System (AmershamPharmacia). Both bands were confirmed to be suc1 by N-terminal sequencing (Department of Biochemistry, University of Cambridge).