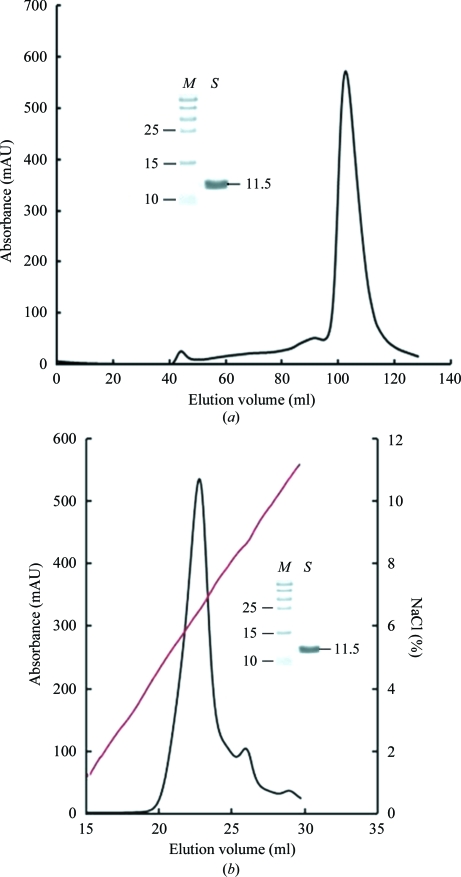
Figure 2.
Purification of refolded ns-β2m by FPLC Superdex 200 16/60 HiLoad gel-filtration and Resouce-Q anion-exchange chromatography (GE Healthcare). (a) Gel-filtration profile of the refolded products. The load rate was 1 ml min−1 and elution was monitored at 280 nm. The inset shows a reduced SDS–PAGE gel (15%) of the corresponding purified protein. Lane M contains molecular-weight markers (labelled in kDa) and lane S contains the sample (ns-β2m protein). (b) The results of further purification of the refolded products by anion-exchange chromatography. The protein was eluted at an NaCl concentration of 5.6–7.0%. The inset shows a reduced SDS–PAGE gel (15%) of the corresponding purified protein. Lanes M and S contain molecular-weight markers (labelled in kDa) and sample, respectively.