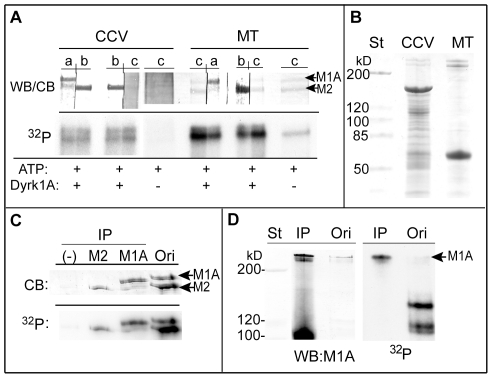
Figure 2. Identification of the phosphorylated protein bands 1 and 2 as MAP1A and MAP2.
(A) MAP1A and MAP2 in the phosphorylated CCVs and MTs. CCVs (20 µg) and purified MTs (5 µg) were incubated with [γ-32P]-ATP with (+) or without (−) GST-Dyrk1A497 as described in Fig. 1, followed by SDS-PAGE without ultracentrifugation. Approximately 9 µg and 2 µg of CCVs and MTs, respectively, were applied per lanes. After transferring proteins, each lane of the PVDF membranes was cut into two strips for immunostaining either with anti-MAP1A (a) or anti-MAP2 (b) antibody, or for Coomassie Blue staining (c). The strips were reassembled (WB/CB) and subjected to autoradiography (32P). (n = 2). (B) Coomassie Blue-staining of the CCV and MT preparations. Ten and five µg of CCVs and MTs, respectively, were applied per lane. (C) Immunoprecipitation of MAP1A and MAP2 from the phosphorylated MTs. MTs (200 µg) were phosphorylated for 1 hr with GST-Dyrk1A497 (18 µg) and 0.2 mM [γ-32P]-ATP in a final volume of 250 µl. After the reaction, the soluble fraction was subjected to immunoprecipitation (IP) by using anti-MAP2 (M2) or anti-MAP1A (M1A) antibody as described in MATERIALS AND METHODS . A negative control for the immunoprecipitation (−) was obtained without primary antibody. The immunoprecipitates were applied to SDS-PAGE followed by Coomassie Blue staining (CB) and autoradiography (32P). (n = 1; various preliminary performances carried out to lead the final assay conditions are not included). Scanning of the MAP1A and MAP2 bands from the original material used for immunoprecipitation (Ori) gave the arbitrary units for these proteins as 3306 and 6323, respectively, whereas those for the radioactivity were 6056 and 12582, respectively. (D) Immunoprecipitation of MAP1A from the extract of the phosphorylated CCVs. CCVs (60 µg) were incubated with GST-Dyrk1A497 (7 µg) and 0.2 mM [γ-32P]-ATP for 1 hr in a final volume of 120 µl. The phosphorylated CCVs were extracted with 0.5 M Tris-HCl, diluted the Tris-HCl concentration, and used for immunoprecipitation with anti-MAP1A antibody (IP). The immunoprecipitates were subjected to blotting (WB) with anti-MAP1A antibody followed by autoradiography (32P). (n = 2). St, pre-stained standard proteins; M1A, MAP1A; M2, MAP2.