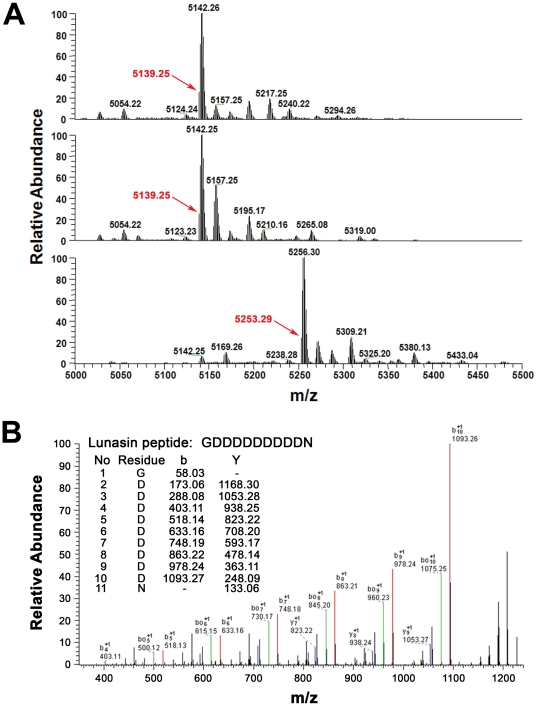
Figure 6. Mass spectrometry of the purified lunasin.
(A, top panel) Deconvoluted MS Spectra of purified lunasin. The monoisotopic mass of the purified lunasin was found to be 5139.25 Da, which is 114.02 Da higher than the expected monoisotopic mass (5025.23 Da) for the 43 amino-acid form of lunasin described in the literature. The mass difference suggests that the predominant form of our purified lunasin contains 44 amino acids and that it contains an additional asparagine residue. (A, middle panel) Deconvoluted spectrum of lunasin reduced with DTT. Reduction with DTT did not cause a mass shift, indicating there is no disulfide bond present in the purified lunasin. (A, bottom panel) Deconvoluted spectrum of lunasin complex treated with DTT and IAA. The monoisotopic mass of lunasin shifted to 5253.29 Da after alkylation with IAA, which is 114.04 Da higher than unalkylated lunasin. This mass shift confirmed that lunasin has two free cysteine residues as expected. (B) MS/MS spectrum of C-terminal peptide of lunasin. Calculated b and Y ions for the peptide GDDDDDDDDDN are shown in the table inset. The matched b (red) and Y (blue) ions detected match very well the expected fragment ion values for this peptide. Signals corresponding to the loss of one (green) or more H2O molecules, which are expected in MS/MS spectra of peptides with multiple acidic residues, are also evident in the spectrum. These [b – H2O] signals are consistent with the presence of the GDDDDDDDDDN peptide. This analysis confirmed that the residue at the C-terminus of lunasin purified from soybean is asparagine rather than aspartic acid.