Abstract
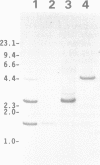
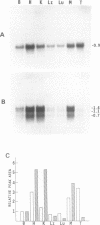
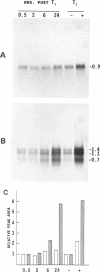
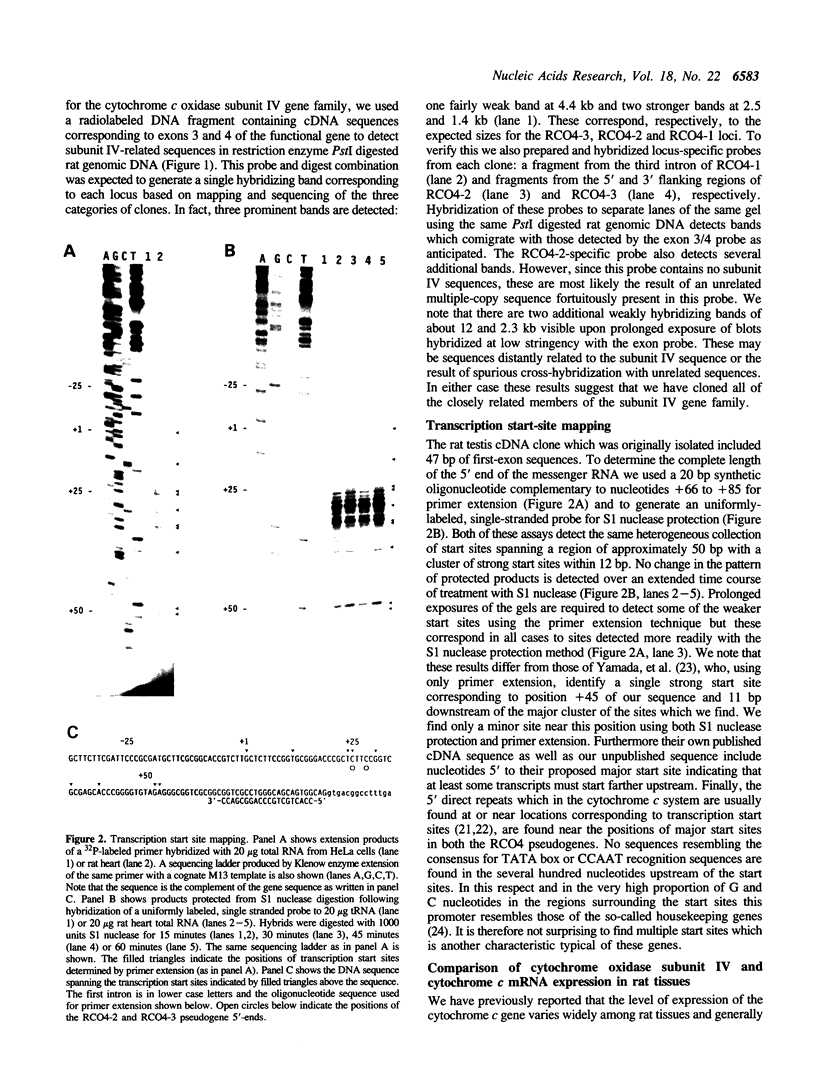
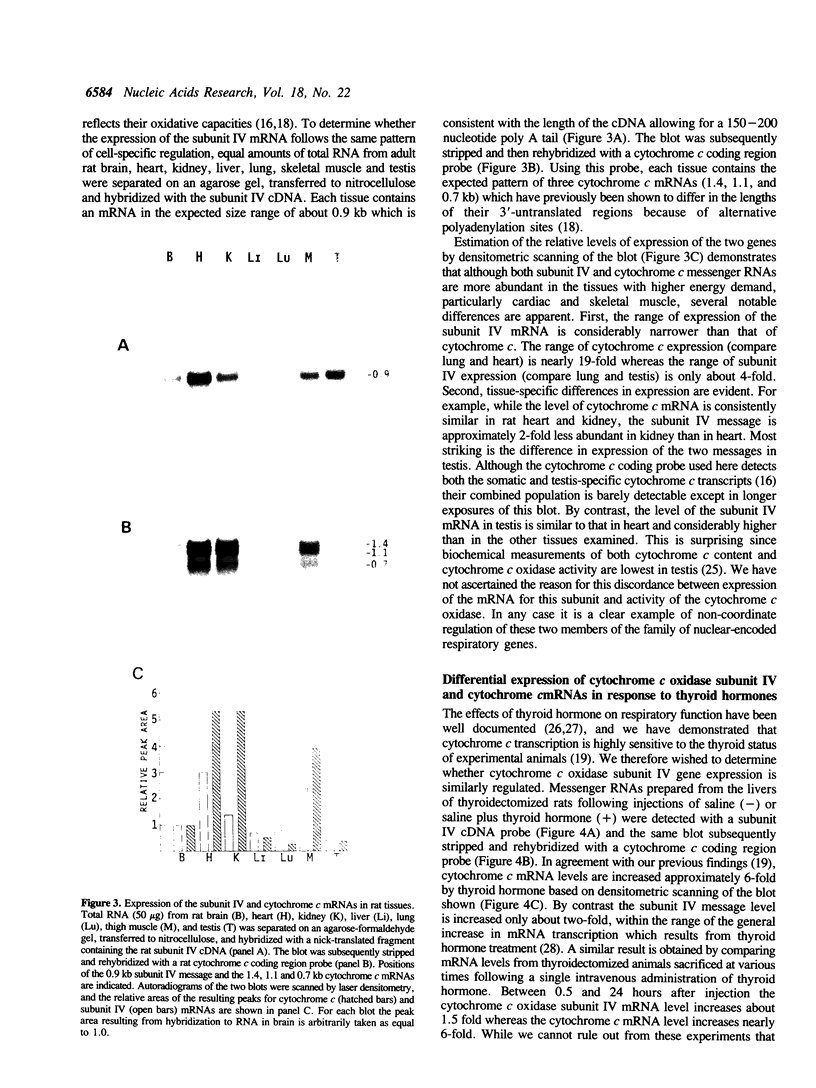
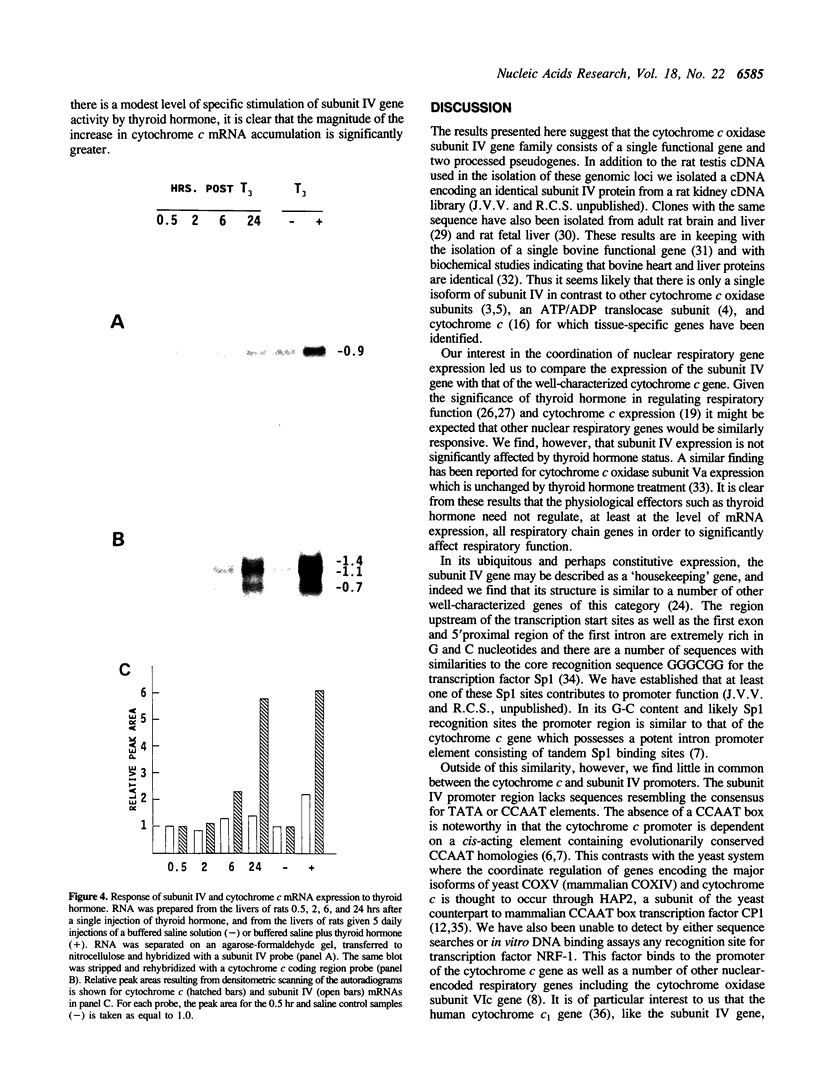
We have isolated three members of the rat cytochrome c oxidase subunit IV gene family: one functional gene and two processed pseudogenes. The pseudogenes appear to represent the only other closely related sequences in this family. The functional gene encodes an isoform which is expressed in all tissues examined and has features characteristic of 'housekeeping' genes. These include multiple transcription start sites mapped to within an approximately 50 bp region and a GC-rich promoter lacking typical CCAAT or TATAA sequences. Although the subunit IV gene is expressed at its highest levels in cardiac and skeletal muscle, consistent with the high energy demand in those tissues, its expression differs from that of cytochrome c in several respects. 1) Subunit IV mRNA abundance in various tissues is relatively uniform when compared to the highly variable levels of cytochrome c mRNAs. 2) Unlike cytochrome c, subunit IV mRNA is expressed at a surprisingly high level in testis. 3) While cytochrome c mRNA levels in liver are increased markedly in response to thyroid hormone treatment, subunit IV mRNA is not significantly affected. Differences in the expression of these two nuclear-encoded respiratory genes are consistent with differences in regulatory elements within their promoters. Therefore, the regulation of nuclear-encoded respiratory genes in response to tissue demands for cellular energy may not be satisfactorily explained by a set of universal regulators common to all such genes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachman N. J., Lomax M. I., Grossman L. I. Two bovine genes for cytochrome c oxidase subunit IV: a processed pseudogene and an expressed gene. Gene. 1987;55(2-3):219–229. doi: 10.1016/0378-1119(87)90282-4. [DOI] [PubMed] [Google Scholar]
- Burke J. F. High-sensitivity S1 mapping with single-stranded [32P]DNA probes synthesized from bacteriophage M13mp templates. Gene. 1984 Oct;30(1-3):63–68. doi: 10.1016/0378-1119(84)90105-7. [DOI] [PubMed] [Google Scholar]
- Chodosh L. A., Olesen J., Hahn S., Baldwin A. S., Guarente L., Sharp P. A. A yeast and a human CCAAT-binding protein have heterologous subunits that are functionally interchangeable. Cell. 1988 Apr 8;53(1):25–35. doi: 10.1016/0092-8674(88)90484-9. [DOI] [PubMed] [Google Scholar]
- Evans M. J., Scarpulla R. C. Both upstream and intron sequence elements are required for elevated expression of the rat somatic cytochrome c gene in COS-1 cells. Mol Cell Biol. 1988 Jan;8(1):35–41. doi: 10.1128/mcb.8.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M. J., Scarpulla R. C. Interaction of nuclear factors with multiple sites in the somatic cytochrome c promoter. Characterization of upstream NRF-1, ATF, and intron Sp1 recognition sequences. J Biol Chem. 1989 Aug 25;264(24):14361–14368. [PubMed] [Google Scholar]
- Evans M. J., Scarpulla R. C. NRF-1: a trans-activator of nuclear-encoded respiratory genes in animal cells. Genes Dev. 1990 Jun;4(6):1023–1034. doi: 10.1101/gad.4.6.1023. [DOI] [PubMed] [Google Scholar]
- Evans M. J., Scarpulla R. C. The human somatic cytochrome c gene: two classes of processed pseudogenes demarcate a period of rapid molecular evolution. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9625–9629. doi: 10.1073/pnas.85.24.9625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalan G., Droste M., Kadenbach B. Nucleotide sequence of cDNA encoding subunit IV of cytochrome c oxidase from fetal rat liver. Nucleic Acids Res. 1989 Jun 12;17(11):4376–4376. doi: 10.1093/nar/17.11.4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y., Amuro N., Okazaki T. Nucleotide sequence of cDNA for rat brain and liver cytochrome c oxidase subunit IV. Nucleic Acids Res. 1989 Apr 11;17(7):2851–2851. doi: 10.1093/nar/17.7.2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatefi Y. The mitochondrial electron transport and oxidative phosphorylation system. Annu Rev Biochem. 1985;54:1015–1069. doi: 10.1146/annurev.bi.54.070185.005055. [DOI] [PubMed] [Google Scholar]
- Horrum M. A., Tobin R. B., Ecklund R. E. Thyroxine-induced changes in rat liver mitochondrial cytochromes. Mol Cell Endocrinol. 1985 Jul;41(2-3):163–169. doi: 10.1016/0303-7207(85)90019-x. [DOI] [PubMed] [Google Scholar]
- Koerner T. J., Hill J., Tzagoloff A. Cloning and characterization of the yeast nuclear gene for subunit 5 of cytochrome oxidase. J Biol Chem. 1985 Aug 15;260(17):9513–9515. [PubMed] [Google Scholar]
- Lightowlers R., Ewart G., Aggeler R., Zhang Y. Z., Calavetta L., Capaldi R. A. Isolation and characterization of the cDNAs encoding two isoforms of subunit CIX of bovine cytochrome c oxidase. J Biol Chem. 1990 Feb 15;265(5):2677–2681. [PubMed] [Google Scholar]
- Powell S. J., Medd S. M., Runswick M. J., Walker J. E. Two bovine genes for mitochondrial ADP/ATP translocase expressed differences in various tissues. Biochemistry. 1989 Jan 24;28(2):866–873. doi: 10.1021/bi00428a069. [DOI] [PubMed] [Google Scholar]
- Power S. D., Lochrie M. A., Poyton R. O. The nuclear-coded subunits of yeast cytochrome c oxidase. III. Identification of homologous subunits in yeast, bovine heart, and Neurospora crassa cytochrome c oxidases. J Biol Chem. 1984 May 25;259(10):6575–6578. [PubMed] [Google Scholar]
- Poyton R. O., Trueblood C. E., Wright R. M., Farrell L. E. Expression and function of cytochrome c oxidase subunit isologues. Modulators of cellular energy production? Ann N Y Acad Sci. 1988;550:289–307. doi: 10.1111/j.1749-6632.1988.tb35344.x. [DOI] [PubMed] [Google Scholar]
- Sacher R., Steffens G. J., Buse G. Studies on cytochrome c oxidase, VI. Polypeptide IV. the complete primary structure. Hoppe Seylers Z Physiol Chem. 1979 Oct;360(10):1385–1392. doi: 10.1515/bchm2.1979.360.2.1385. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpulla R. C., Agne K. M., Wu R. Isolation and structure of a rat cytochrome c gene. J Biol Chem. 1981 Jun 25;256(12):6480–6486. [PubMed] [Google Scholar]
- Scarpulla R. C., Kilar M. C., Scarpulla K. M. Coordinate induction of multiple cytochrome c mRNAs in response to thyroid hormone. J Biol Chem. 1986 Apr 5;261(10):4660–4662. [PubMed] [Google Scholar]
- Scarpulla R. C. Processed pseudogenes for rat cytochrome c are preferentially derived from one of three alternate mRNAs. Mol Cell Biol. 1984 Nov;4(11):2279–2288. doi: 10.1128/mcb.4.11.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpulla R. C., Wu R. Nonallelic members of the cytochrome c multigene family of the rat may arise through different messenger RNAs. Cell. 1983 Feb;32(2):473–482. doi: 10.1016/0092-8674(83)90467-1. [DOI] [PubMed] [Google Scholar]
- Schlerf A., Droste M., Winter M., Kadenbach B. Characterization of two different genes (cDNA) for cytochrome c oxidase subunit VIa from heart and liver of the rat. EMBO J. 1988 Aug;7(8):2387–2391. doi: 10.1002/j.1460-2075.1988.tb03083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H., Hosokawa Y., Nishikimi M., Ozawa T. Structural organization of the human mitochondrial cytochrome c1 gene. J Biol Chem. 1989 Jan 25;264(3):1368–1374. [PubMed] [Google Scholar]
- TATA J. R., ERNSTER L., LINDBERG O., ARRHENIUS E., PEDERSEN S., HEDMAN R. The action of thyroid hormones at the cell level. Biochem J. 1963 Mar;86:408–428. doi: 10.1042/bj0860408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trueblood C. E., Wright R. M., Poyton R. O. Differential regulation of the two genes encoding Saccharomyces cerevisiae cytochrome c oxidase subunit V by heme and the HAP2 and REO1 genes. Mol Cell Biol. 1988 Oct;8(10):4537–4540. doi: 10.1128/mcb.8.10.4537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Itallie C. M. Thyroid hormone and dexamethasone increase the levels of a messenger ribonucleic acid for a mitochondrially encoded subunit but not for a nuclear-encoded subunit of cytochrome c oxidase. Endocrinology. 1990 Jul;127(1):55–62. doi: 10.1210/endo-127-1-55. [DOI] [PubMed] [Google Scholar]
- Virbasius J. V., Scarpulla R. C. Structure and expression of rodent genes encoding the testis-specific cytochrome c. Differences in gene structure and evolution between somatic and testicular variants. J Biol Chem. 1988 May 15;263(14):6791–6796. [PubMed] [Google Scholar]
- Williams R. S., Garcia-Moll M., Mellor J., Salmons S., Harlan W. Adaptation of skeletal muscle to increased contractile activity. Expression nuclear genes encoding mitochondrial proteins. J Biol Chem. 1987 Feb 25;262(6):2764–2767. [PubMed] [Google Scholar]
- Yamada M., Amuro N., Goto Y., Okazaki T. Structural organization of the rat cytochrome c oxidase subunit IV gene. J Biol Chem. 1990 May 5;265(13):7687–7692. [PubMed] [Google Scholar]
- Yanamura W., Zhang Y. Z., Takamiya S., Capaldi R. A. Tissue-specific differences between heart and liver cytochrome c oxidase. Biochemistry. 1988 Jun 28;27(13):4909–4914. doi: 10.1021/bi00413a048. [DOI] [PubMed] [Google Scholar]
- Zeviani M., Nakagawa M., Herbert J., Lomax M. I., Grossman L. I., Sherbany A. A., Miranda A. F., DiMauro S., Schon E. A. Isolation of a cDNA clone encoding subunit IV of human cytochrome c oxidase. Gene. 1987;55(2-3):205–217. doi: 10.1016/0378-1119(87)90281-2. [DOI] [PubMed] [Google Scholar]