Table IV.
Predictive Approaches of Human Plasma Concentration–Time Profile
| Method | Equation | Data required | Dataset | <twofold |
|---|---|---|---|---|
| Species-invariant time method (90) |
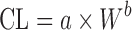
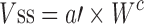
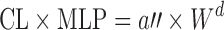
|
Plasma concentration–time profiles, dose, CL, and Vdss in at least two animal species | All categories | |
Equivalent time: 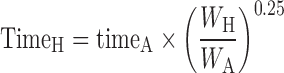
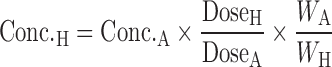
|
n = 2 (100) | 100% (AUC) | ||
Kallynochrons: 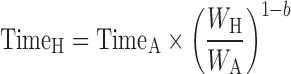
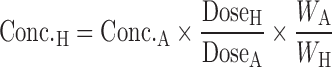
|
n = 3 (90) | 67% (CL) | ||
Apolysichrons: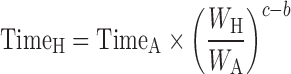
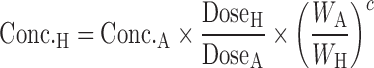
|
||||
Dienetichrons: 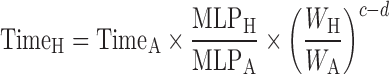
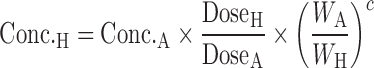
|
||||
| C ss–MRT method (91,92) |
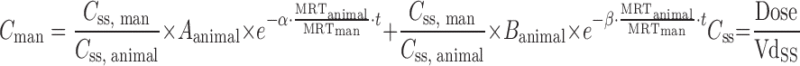
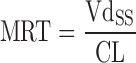
|
Plasma concentration–time profiles, dose, CL, and Vdss in at least two animal species | n = 4 (92) | 75% (AUC) |
| 75% (C max) | ||||
| 50% (C min) | ||||
| 100% (t 1/2) | ||||
| n = 4 (91) | 100% (CL) | |||
| 50% (CL) | ||||
| 75% (Vdss) | ||||
| Human PK prediction using PBPK model (95,96) | Predict K
p (tissue composition-based method) or experimentally measure in rats and assume K
p, u, rat = K
p, u, human. For hepatically eliminated compounds: CLH is scaled from microsome or hepatocyte data. For renally eliminated compounds: 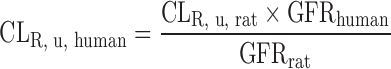 . Oral absorption: GastroPlusTM
. Oral absorption: GastroPlusTM
|
Blood flow, in vitro intrinsic clearance, tissue-to-plasma ratios, B/P ratio, fraction unbound in plasma, solubility and permeability information | n = 19 (96) | 83% (CL) |
| 50% (Vdss) | ||||
| 75% (t 1/2) | ||||
| 92% (AUC) | ||||
| 67% (C max) | ||||
| 100% (t max) |
