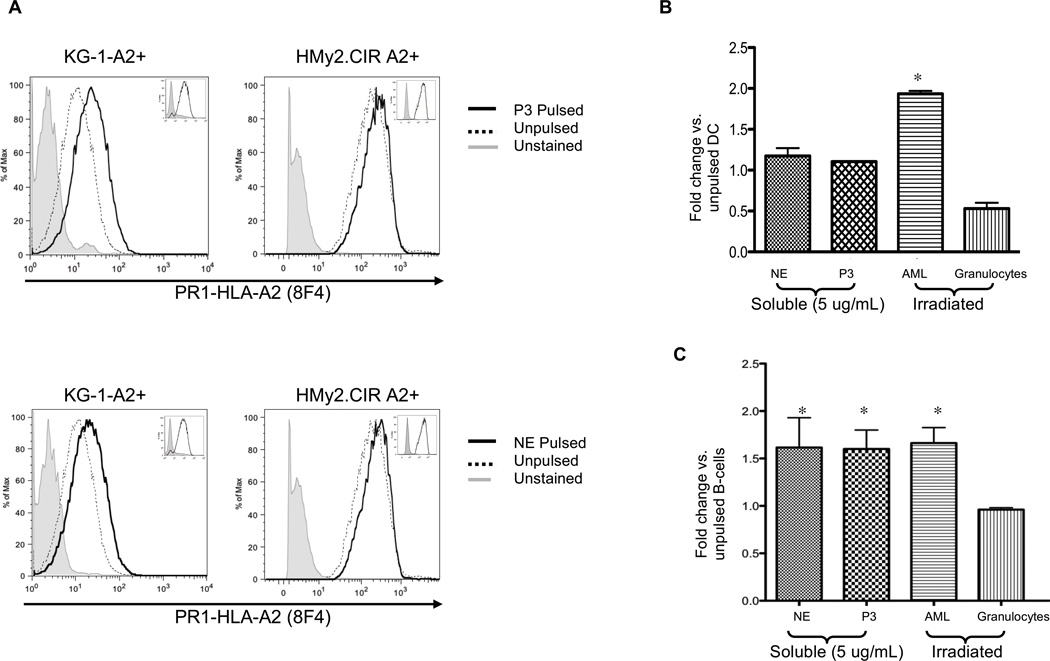
Figure 4. Leukemia, mDCs and B-cells cross-present soluble and cell-associated NE and P3.
(A) A2-transfected KG-1 (KG-1-A2+) and -HMy2.CIR (HMy2.CIR A2+) leukemia cell lines were pulsed with NE or P3 (5 µg/mL) for 6 and 18 hours, respectively. Cells were stained with Alexa-647 conjugated 8F4 antibody and analyzed by flow cytometry. Results show increased PR1 presentation following cell pulsing. Insets show no change in HLA-A2 expression following NE or P3 pulsing. Results are representative of 3 separate experiments. (B) HLA-A2 positive normal mDCs or (C) B-cells were cultured with soluble NE (5 µg/mL), P3 (5 µg/mL), or irradiated leukemia (n=4) or granulocytes (n=3) at a 1:1 APC to irradiated-cell ratio for 6 hours. Cells were stained with Alexa-647-conjugated 8F4 antibody. CD33, CD34, CD38, HLA-A2, aqua live/dead stain and light scatter were used to exclude irradiated cells. CD19+ and CD11c+/CD14− were used to gate on B-cells and mDCs, respectively. (A–C) Since 8F4 binds PR1/HLA-A2, in which the HLA-A2 molecule makes up a large portion of the conformational epitope,19, 34 8F4 antibody bound HLA-A2 on unpulsed cells. However, greater staining in pulsed cells indicates PR1 cross-presentation. Results represent duplicate wells from 3 representative experiments. All data points are expressed as mean fold-change ± SD in the MFI of 8F4 staining compared with unpulsed cells. ANOVA followed by Tukey tests were performed using Prism 5.0 software (*P<0.05).