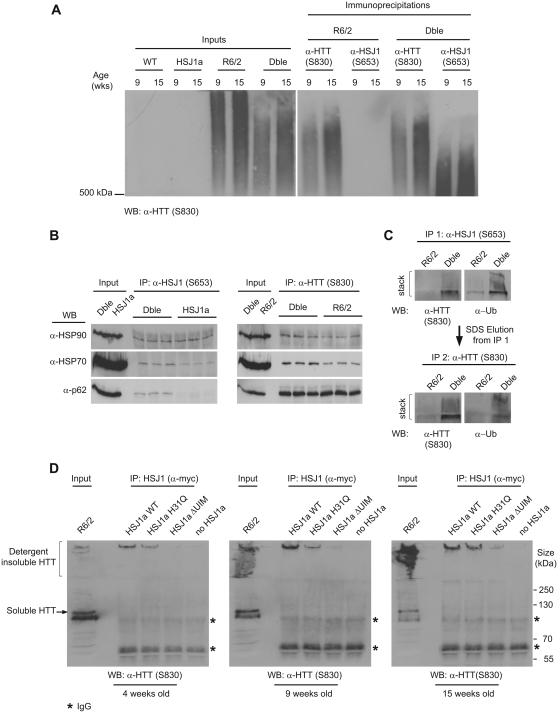
Figure 5.
HSJ1a associates with high molecular weight mutant huntingtin complexes via a UIM-dependent mechanism. (A) Western blot of aggregated material isolated by anti-huntingtin (S830) or anti-HSJ1 (S653) immunoprecipitation and resolved by agarose gel electrophoresis resolution of aggregates. Immunoprecipitations were performed on 9- and 15-week brain tissue from HSJ1a, R6/2 or double transgenic (Dble) mice and mutant huntingtin was detected with S830. (B) Western blots showing co-immunoprecipitation of major regulators of protein homoeostasis from 15-week R6/2 and double transgenic brain tissue after immunoprecipitation with anti-huntingtin (S830) or anti-HSJ1 (S653) antibodies. (C) HSJ1 (S653) immunoprecipitation was performed on 15-week R6/2 or double transgenic brain lysates. Immunoprecipitation complexes were eluted from magnetic beads in denaturing buffer and subjected to a second immunoprecipitation with S830. Levels of ubiquitylated detergent insoluble material after each immunoprecipitation were determined by western blotting and immuno-detection with anti-huntingtin (S830) and anti-Ubiquitin (α-Ub) antibodies. (D) Western blots probed with S830 after HSJ1 immunoprecipitation (anti-myc) from 4-, 9- and 15-week R6/2 brain lysates incubated with lysates from SK-N-SH cells expressing myc-tagged wild-type, J-domain inactive (H31Q) or UIM inactive (ΔUIM) HSJ1a (asterisks denote IgG bands).