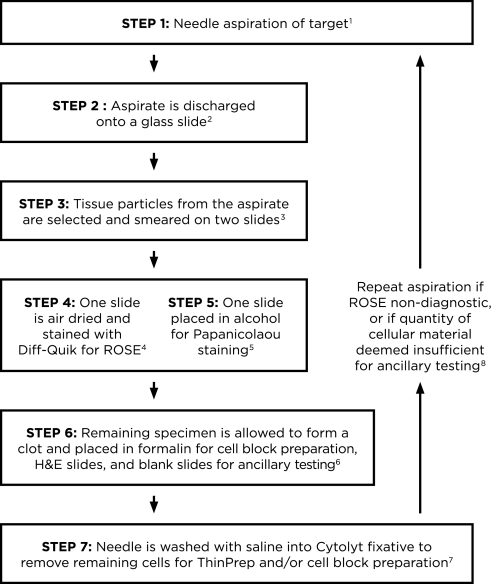
Figure 1.
Algorithm for processing endoscopic ultrasound transbronchial needle aspiration (EBUS-TBNA) specimens. 1Use of the 21-gauge needle may improve diagnostic yield and may provide better preservation of histologic structure compared with the 22-gauge needle. 2The aspirate from the needle is discharged onto a glass slide by blowing air with a 20-ml syringe. If there is resistance because of clot formation, and material cannot be expelled by air, the stylet is reinserted to propel the material onto the slide. The specimen is usually placed in its entirety onto a glass slide; it may be expelled onto two slides if there is excessive blood. 3A few tissue particles, which are often tan–white, are selected from the expelled material to make smears. If the specimen has clotted, the clot is gently pressed between two slides to expose tissue particles, which are then smeared onto slides. Note that although a clot can be smeared, its thickness does not allow cells within it to be visualized on a smear. 4One smear is air-dried completely for on-site Diff-Quik staining and rapid on-site cytologic evaluation (ROSE). 5The second smear is fixed in alcohol immediately for Papanicolaou staining in the laboratory. Any air-drying creates artifacts that preclude accurate interpretation. 6The remaining specimen is allowed to clot and is placed in formalin to create a cell block for hematoxylin and eosin (H&E) slides and blank slides for immunocytochemistry, molecular, or fluorescence in situ hybridization testing, if indicated. If lymphoma is suspected on clinical grounds or after ROSE, the remainder of the specimen is placed in RPMI or saline for flow cytometry analysis. An aliquot is placed in saline for cultures in cases where granulomas are noted or infection is suspected. 7Saline (∼1 ml) is passed through the needle into Cytolyt fixative to remove any additional cells for ThinPrep or cell block preparation. 8In addition to assessing diagnostic adequacy, ROSE must include an overall quantitative assessment of the amount of cellular material triaged for cell block and ancillary testing (immunohistochemistry and molecular testing). If this is considered to be potentially insufficient, the needle aspiration should be repeated from the diagnostic location.