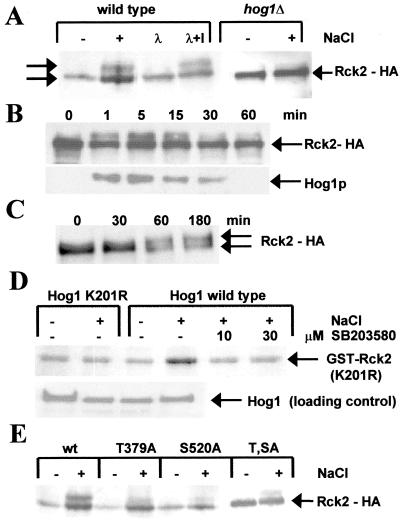
Figure 2.
Phosphorylation of Rck2 by Hog1 in vivo and in vitro. (A) Western Blot of HA epitope-tagged Rck2 before (−) and 10 min after (+) a mild osmotic shock with 0.4 M NaCl. The two forms of Rck2 are indicated by arrows. Samples from lane 2 (+) were treated with λ phosphatase without (λ) and with inhibitor (λ + I). (B) Time course of Rck2 phosphorylation after osmotic shock. HA epitope-tagged Rck2 was assayed by Western blot analysis at the indicated time points. (Lower) Double-phosphorylated Hog1 is detected with the p38 antibody (New England Biolabs). (C) Rck2 phosphorylation after induction of the HOG pathway by galactose-induced expression of a N-terminally truncated SSK2 allele. Samples taken at the indicated time points after induction were assayed by Western blot analysis by using the HA antibody. (D) In vitro kinase assays with bacterially expressed glutathione S-transferase-Rck2 (kinase inactive K201R mutant) and immunoprecipitated Hog1 kinase. C-terminally HA epitope-tagged Hog1 was precipitated from yeast cells before (−) and after (+) activation by 0.4 M NaCl for 5 min, and 32P incorporation was detected by autoradiography. The kinase inactive (K52R) Hog1 mutant was compared with wild type. Additionally, wild-type Hog1 kinase activity was blocked by the specific p38 inhibitor SB 203580 (Calbiochem) at the indicated concentrations. (E) Phosphorylation of Rck2 mutant proteins. The phosphorylation site mutants T379A, S520A, and T379A S520A were tested for phosphorylation, as described for A, by Western blot analysis after a mild osmotic shock (0.4 M NaCl) for 10 min.