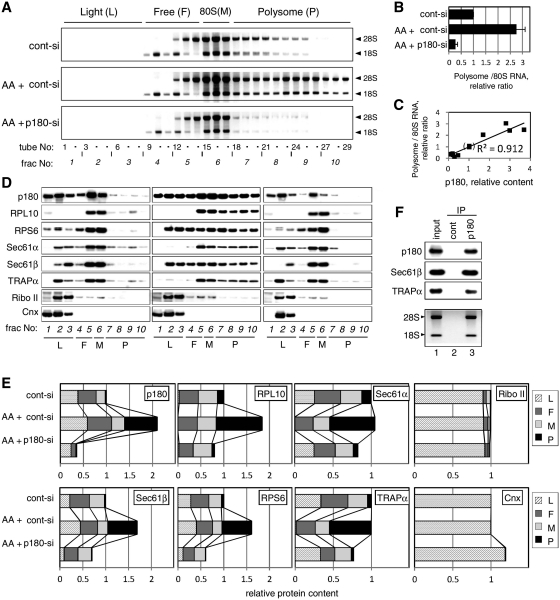
Figure 2.
p180 facilitates polysome assembly on the ER membrane. HEL cells were cultured in the presence or absence of ascorbate (AA) and treated with a control scrambled (cont-si) or p180-specific (p180-si) siRNA. At post-transfection Day 4, sequential digitonin extractions were carried out to obtain the membrane fractions, followed by polysome analysis using a 15–50% sucrose gradient. Equal amounts of samples normalized by the total DNA contents were subjected to the polysome analysis. (A) The ribosomal RNAs in each fraction were analyzed by agarose electrophoresis. The positions of the polysome (P), monosome (M), free 40S and 60S subunits (F) and light (L) fractions are shown at the top. Top panel: cont-si-treated cells without ascorbate; middle panel: cont-si-treated cells with ascorbate; bottom panel: p180-si-treated cells with ascorbate. (B) The total RNA contents of the polysome fractions were estimated by densitometric scanning of the gels, and the relative ratios of polysomes to 80S monosome are shown (means ± SD, n = 4). (C) The relative amounts of p180 and polysomes in the membrane fractions were plotted to evaluate their correlation. The 12 samples used were obtained from five separate experiments under various conditions. The values for cont-si-treated cells without ascorbate stimulation were set as 1. (D) The distributions of p180 and other translocon-related proteins in the collected fractions were analyzed by western blotting. Non-stimulated cells with cont-si (left), ascorbate-stimulated cells with cont-si (middle), and ascorbate-stimulated p180-si treated cells (right) are shown. (E) Quantitative data for the protein markers in the polysome (P), monosome (M), free 40S and 60S subunits (F) and light (L) fractions are shown. The total amounts of each protein in the membrane fractions were estimated by densitometry using samples prior to sucrose density centrifugation, and their relative contents were assigned to the P, M, F and L fractions by densitometric scanning of the data shown in panel D. The total amounts for the membrane fractions from the cont-si-treated cells without ascorbate stimulation were set as 1. (F) Polysome fractions were collected from the cont-si treated cells after ascorbate stimulation and subjected to IP assays using an anti-p180 antibody or control rabbit IgG bound to magnetic beads conjugated with sheep anti-rabbit IgG. The samples captured by the beads were immunoblotted with antibodies against marker proteins or analyzed for rRNAs. Lane 1, input samples; lane 2, immunoprecipitates with control IgG; lane 3, immunoprecipitates with anti-p180 antibody. Sec61α was not visible because of co-existing immunoglobulin chains.