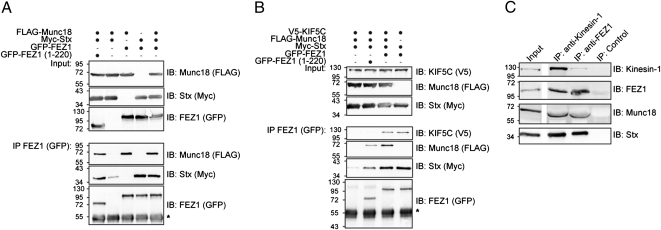
Fig. 2.
Protein complexes formed by FEZ1 reveal its function as cargo adaptor for Kinesin-1 (KIF5C). (A and B) HEK 293 cells expressing combinations of tagged versions of FEZ1, Munc18, KIF5C, or Stx were immunoprecipitated (IP) using anti-GFP antibodies and immunoblotted (IB) using tag-specific antibodies (anti-FLAG, anti-GFP, anti-Myc, or anti-V5). Molecular mass markers are indicated are in kilodaltons. *Ig heavy chain. (A) FEZ1 forms a trimeric complex with Munc18 and Stx. Full-length and FEZ1 (amino acids 1–220) efficiently immunoprecipitate Stx and Munc18 indicating that direct binding of Munc18 to FEZ1 is not necessary for trimeric complex formation. (B) KIF5C, FEZ1, Stx, and Munc18 can be concurrently isolated as a complex. In addition, binding of Stx to KIF5C can occur without Munc18 but is dependent on the presence of the coiled-coil domain of FEZ1. (C) The FEZ1/Kinesin-1 transport complex comprising FEZ1, Stx, Munc18, and Kinesin-1 can be immunoisolated from rat brain postnuclear supernatants using anti-FEZ1 or anti-Kinesin-1 antibodies.