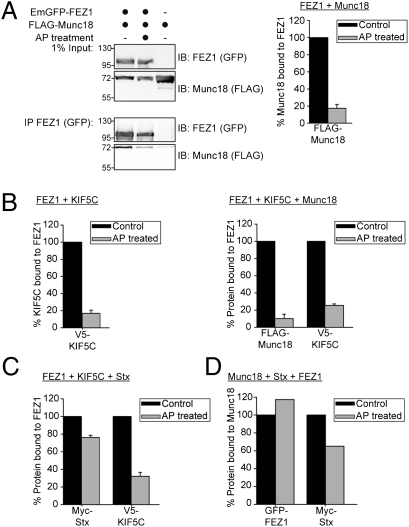
Fig. 5.
Phosphorylation of FEZ1 regulates binding to its interaction partners. HEK 293 cell lysates expressing combinations of tagged versions of FEZ1, Munc18, KIF5C, or Stx treated with or without AP were immunoprecipitated using tag-specific antibody (anti-GFP) and immunoblotted using tag-specific antibodies (anti-FLAG, anti-GFP, anti-Myc, or anti-V5). The amount of immunoprecipitated protein was determined by densitometry, with the untreated sample serving as reference (100%). A representative Western blot is shown in A. Data in A–C were obtained from three independent experiments. Error bars represent SDs. (A) Treatment of cell lysates with AP significantly reduces Munc18 binding to FEZ1. (B) Phosphorylation regulates assembly of the trimeric FEZ1-Munc18-Kinesin-1 complex. Dephosphorylation of FEZ1 reduced its binding to KIF5C (left chart) and dissociates the ternary complex of FEZ1, Munc18, and KIF5C (right chart). (C) Dephosphorylation reduces the amount of KIF5C bound to FEZ1 but significant amounts of Stx remain bound to FEZ1. (D) Significant amounts of FEZ1-Stx-Munc18 complexes remain isolatable even after AP treatment. HEK 293 cell lysates expressing tagged versions of Munc18, Stx, and FEZ1 treated with or without AP were immunoprecipitated with tag-specific (anti-FLAG) antibody and immunoblotted using tag-specific antibodies (anti-FLAG, anti-Myc, or anti-GFP). Data were obtained from the average of two independent experiments.