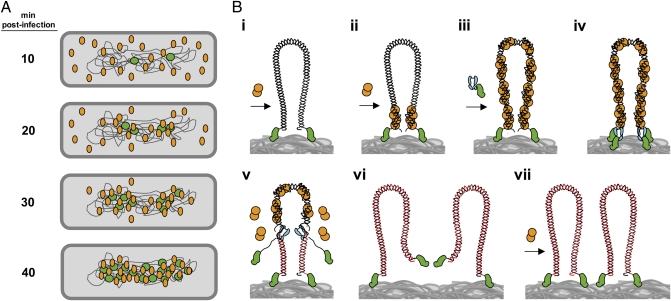
Fig. 5.
Model of nucleoid-associated ϕ29 DNA replication and recruitment of protein p6 to the viral genome. (A) Once ϕ29 infection takes place, the TP-DNA genomes (green circles) are recruited to the bacterial nucleoid. Soon after the injection process, early promoters mediate a rapid expression of protein p6 (orange ovals). At 10 min post infection, p6 is shown to distribute following a peripheral helix-like pattern. Later, and simultaneously with the generation of new replicated ϕ29 genomes, the bulk of protein p6 is recruited to the bacterial nucleoid. (B, i) A ϕ29 TP-DNA molecule (linear dsDNA drawn as a double helix) is shown attached to the bacterial nucleoid surface (gray mass at bottom) by the parental TPs (green). Protein p6 (orange circles) is recruited to the bacterial nucleoid by binding to the viral DNA. (B, ii) Protein p6 binds the origins of replication of the ϕ29 genome through its DNA-binding domain, forming a nucleoprotein complex that helps to open the DNA ends, facilitating the initiation step of replication. (B, iii) The p6-bound origins act as a nucleation site from which more p6 molecules bind to the rest of the viral genome. (B, iv) The heterodimer formed by TP and DNA polymerase (cyan) recognizes the p6-bound replication origins and initiates DNA replication. (B, v) After a transition step, the DNA polymerase dissociates and continues processive elongation of the nascent DNA strands (red lines) coupled to strand displacement, removing p6 molecules from the viral genome during the polymerization process. (B, vi and vii) Once DNA replication is completed, two ϕ29 TP-DNA molecules are ready for another round of replication. Proteins, viral DNA, and bacterial nucleoid are not drawn to scale.