Fig. P1.
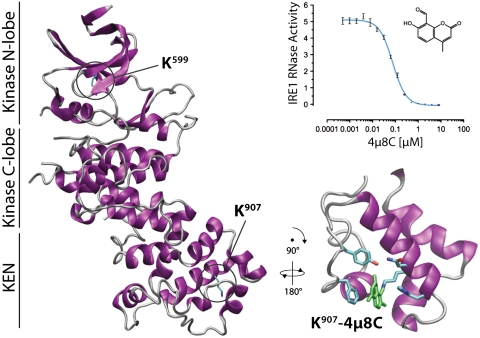
Targeted inhibition of IRE1. 8-formyl-7-hydroxy-4-methylcoumarin (abbreviated 4μ8C, see inset to graph), identified by high-throughput screening, was found to inhibit the endonuclease activity of mammalian IRE1 with high selectivity in both an in vitro FRET-derepression assay (see graph) and cultured cells. The compound binds to a critical lysine in the endonuclease active site of IRE1 by formation of an unusually stable Schiff base. IRE1 K907-4μ8C modification constrains the flexibility of the endonuclease site by formation of stacking interactions with F889 and interjects between essential catalytic residues, inactivating the enzyme. Structure shows human IRE1 protomer (Protein Data Bank ID code 3P23, Left) and the detail (Lower Right, residues 870 to 939) shows computationally docked 4μ8C (green) at K907, with residues F889, Y892, N906, K907, and H910 highlighted.