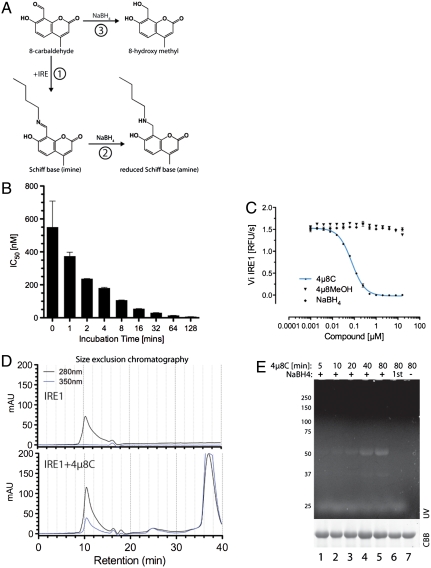
Fig. 2.
Stable Schiff base formation between 4μ8C and IRE1. (A) Predicted reaction schemes for Schiff base formation of 4μ8C to form a 4μ8C-lysyl imine (in reaction 1) that can be reduced to the stable lysyl amine by borohydride (NaBH4, reaction 2). Reductive inactivation of 4μ8C to the 8-hydroxymethyl is depicted in reaction 3. (B) Plot of the relationship between the incubation time of IRE1 with 4μ8C and the apparent IC50 in the in vitro RNase assay (mean ± SEM, n = 2). (C) In vitro RNase activity of IRE1 following incubation with 4μ8C and 4μ8C that had been previously reduced with borohydride (NaBH4; mean ± SEM, n = 2). (D) Absorbance trace of material eluting from a size exclusion chromatography column loaded with IRE1 or IRE1 and 200 μM 4μ8C. Note the emergence of a novel absorbance peak at 350 nm that comigrates with IRE1 in the 4μ8C-treated sample. (E) Fluorescent micrograph (excitation 340 nm, emission 450 nm) of an SDS-PAGE loaded with IRE1, following exposure 120 μM 4μ8C for the indicated times with or without subsequent reduction by borohydride. The sample in lane 6 was reacted with 4μ8C that had been previously reductively inactivated by borohydride.