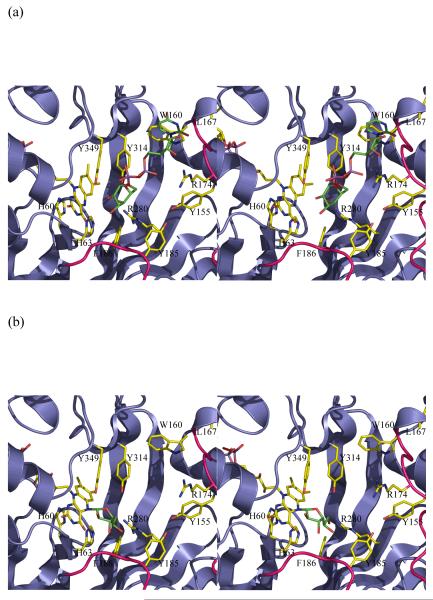
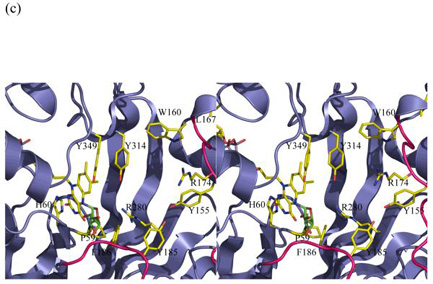
Figure 3.
Stereo images of models of substrate with mutase
(a) The initial complex between UDP-galactose of the active reduced form of the K. pneumoniae enzyme. This complex is predicted to occur in a mechanism involving electron transfer or a covalent intermediate. No significant re-arrangements are required to accommodate the substrate. The structurally diverse loop 5 is shown in pink.
(b) A model of the covalent adduct with the re-face buckle of isoalloxazine ring. The re-face buckled isoalloxazine ring is taken from a thioredoxin structure 17. This model allows interactions with key conserved residues. The model would require conformation changes in side chain positions only to avoid steric clashes.
(c) The covalent adduct based on the experimental K. pneumoniae FADH− structure. The sugar is interpenetrating with the protein structure. Either FADH− adopts a different buckle in the presence of substrate or the protein undergoes a profound conformation change. His 63 has been omitted for clarity and Pro59 added to this figure.