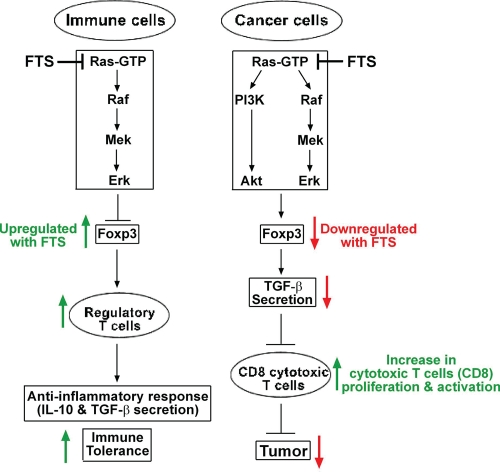
Figure 6. Proposed mechanism explaining the differential effects of Ras inhibition on immune and cancer cells.
Ras inhibition by FTS acts differently on Foxp3 expression in immune cells and in cancer cells. In immune cells, Ras regulates Foxp3 expression via the MAPK pathway. Its inhibition results in upregulation of Foxp3 expression, thereby augmenting Foxp3+ regulatory T cells (Tregs) [3-4]. The upregulated CD25+Foxp3+ Tregs induce an anti-inflammatory effect by secreting tolerogenic cytokines, such as IL-10 and TGF-β, which attenuate the proliferation of effector T cells and thus help to maintain immune tolerance [42]. In the glioma cancer cells, Ras regulates Foxp3 expression via both the MAPK and the PI3K pathways. Treatment of the cancer cells with FTS results in downregulation of Foxp3, which in turn attenuates expression of the immunosuppressive cytokine TGF-β from glioma cells. A similar effect of Foxp3 on TGF-β expression levels has been demonstrated in melanoma cells [43]. The decrease in TGF-β produces an inflammatory tumor microenvironment, which promotes robust proliferation, migration, and activation of antitumor CTLs. This effect on the CTLs intensifies their antitumor character, resulting in decreased tumor growth.