Abstract
Background/Aims
Newly developed and potent antiviral agents suffer from the problem of drug resistance. Multidrug resistance is a major impediment in the treatment of patients with chronic hepatitis B (CHB). In line with American Association for the Study of Liver Diseases guidelines, adefovir dipivoxil (ADV) add-on therapy is recommended in the case of lamivudine resistance, while tenofovir disoproxil fumarate (TDF) is recommended for ADV or entecavir (ETV) resistance. TDF is currently not available in Korea. ADV+ETV combination therapy may be a viable alternative to TDF in patients with either ADV or ETV resistance. However, the efficacy of ADV+ETV combination therapy in patients with CHB and multidrug resistance is unclear. This study investigated the efficacy of ADV+ETV combination therapy in patients with multidrug resistance.
Methods
Twenty-five patients were enrolled and were administered ADV+ETV combination therapy for at least 6 months. Blood was drawn at baseline and at 3, 6, 9, and 12 months after commencing treatment, and the following blood parameters were analyzed: alanine transaminase, hepatitis B e-antigen (HBeAg), anti-hepatitis B e-antigen, and hepatitis B virus (HBV) DNA levels. The initial virological response (IVR) was defined as an HBV DNA level of <4 log10 copies/mL after 6 months of combination therapy.
Results
The IVR rate was 76%. The proportion of patients with a high viral load (≥5.0 log) dropped from 76% at baseline to only 5% after 6 months of treatment. The biochemical response rate during the first 6 months was 71%. HBeAg was lost in 2 patients (10%).
Conclusions
ADV+ETV combination therapy induced a good IVR in CHB patients who were refractory to more than 2 antiviral agents. This regimen may be a good alternative to TDF in Korea, where that drug is not available.
Keywords: Adefovir, Entecavir, Combination drug therapy, Drug resistance, Treatment efficacy
INTRODUCTION
About 350 million people world-wide are chronically infected with hepatitis B (HBV),1,2 which can result in severe liver disease that eventually progresses to cirrhosis and hepatocellular carcinoma.3,4 To date, interferon alpha and five nucleos(t)ide analogs (NAs), namely, lamivudine (LAM), adefovir (ADV), entecavir (ETV), clevudine and telbivudine have been approved for the treatment of chronic hepatitis B (CHB) in Korea. In South-East Asia, oral nucleosides are prescribed more frequently than interferon because genotype C is common.5 Drug resistance often results from prolonged oral nucleoside treatment. LAM was first released for general use in Korea in 1999. Thereafter, the remaining four antiviral agents were introduced in a sequential manner. ETV resistance occurs frequently in patients with LAM resistance.6 Moreover, LAM-resistant patients with CHB sometimes also exhibit ADV resistance, although ADV is thought to be a good rescue therapy for LAM resistance.7 In addition, clevudine (CLV) and telbivudine (LdT) are very similar to LAM in terms of their chemical structure and resistance profile. Thus, an alternative treatment for patients with ADV or ETV resistance is needed. One possible alternative is ADV-ETV combination therapy. This approach has the advantage that the drugs may synergize in terms of efficacy while preventing the emergence of mutants. Its disadvantage is its high cost.
The present study investigated the efficacy of ADV-ETV combination therapy in patients with CHB who did not respond to ADV add-on or high dose (1 mg) ETV therapy. In addition, factors that promoted an initial virological response (IVR) to the combination therapy were identified.
MATERIALS AND METHODS
Subjects
In total, 25 patients with nucleoside-refractory CHB were enrolled in the study. All patients exhibited virological breakthrough (VBT) before starting combination therapy, as defined by an increase in HBV DNA levels of at least 1 log10copies/mL (log) from the nadir during rescue therapy such as ADV add-on and high-dose ETV therapy. Patients were considered to be refractory if they had an inadequate virological response with or without documented genotypic mutations while they were receiving antiviral therapy. An inadequate virological response was defined as an HBV DNA level >4 log at 6 months of rescue therapy. The patients were followed up regularly at 3-month intervals.
The inclusion criteria were as follows: patients were positive for hepatitis B surface antigen (HBsAg) at least 1 year before commencing combination therapy, had HBV DNA level >4 log, and received both ADV and ETV once daily for at least 6 months. Several biochemical parameters and HBV DNA levels were tested at baseline and every 3 months during combination therapy.
None of the patients were co-infected with hepatitis C virus or human immunodeficiency virus or concomitant liver diseases such as alcoholic liver disease or autoimmune liver disease. The IVR was defined as HBV DNA levels below 4 log at 6 months of combination therapy. The continued virological response (CVR) was defined as HBV DNA levels below 4 log after the 6 month time point.
Analysis of virological markers
Routine biochemical tests were performed during therapy by using standard procedures. HBsAg, hepatitis B e-antigen (HBeAg), and anti-hepatitis B e-antigen (anti-HBe) were tested by using a commercial radioimmunoassay kit (Abbott Laboratories, Chicago, IL, USA). HBV DNA levels were determined quantitatively by real time PCR (Roche Molecular Systems, Branchburg, NJ, USA) that had a detection limit of 70 copies/mL. The HBV genotype was not examined because all patients were known to have genotype C.8,9
Genotypic analysis
All baseline patient samples were analyzed for genotypic resistance before starting the combination therapy and at the end of treatment. On-treatment was defined as the time point just before starting the ADV-ETV combination therapy. Off-treatment was defined as the end of combination therapy. Moreover, when VBT was observed, patient samples were analyzed again to identify the mutations that were associated with the genotypic resistance. Genotypic resistance was investigated by restriction fragment mass polymorphism analysis, as described previously by Lee et al (Green-Cross Medical Laboratories, Giheung, Korea).10
Statistical analysis
Statistical testing was performed by using SPSS version 12 (SPSS Inc., Chicago, IL, USA). The data are reported as median (range). The HBV DNA data were logarithmically transformed prior to analysis. Continuous variables were compared by using the Mann-Whitney U test and Student's t test. Categorical data were compared by using the Pearson χ2 test or Fisher's exact test. The cumulative probability of achieving CVR was estimated by using the Kaplan-Meier method. Factors associated with an IVR were analyzed by univariate and multivariate analysis. A P-value less than 0.05 was considered to be statistically significant.
RESULTS
Baseline characteristics
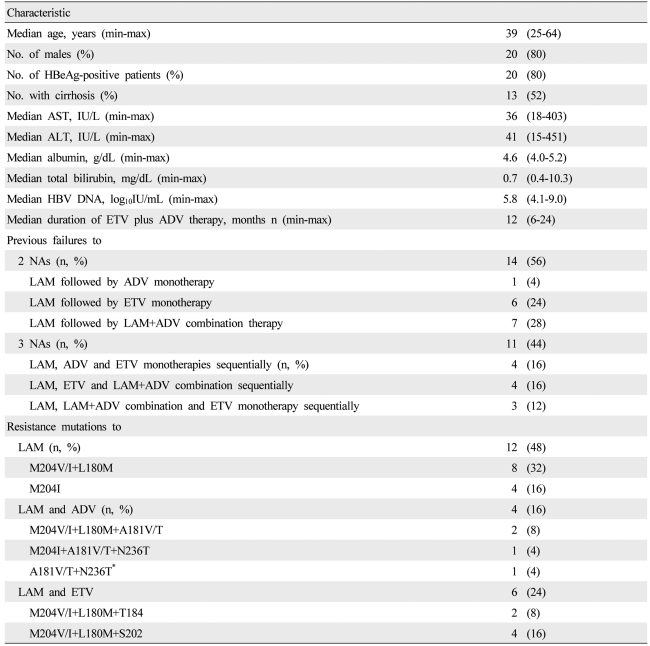
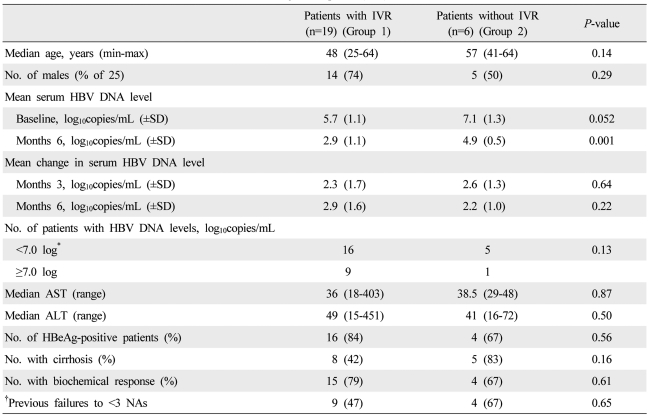
The study population comprised 25 nucleoside-refractory patients; with CHB who had previously shown established drug resistance or persistent VBT in at least two measurements. Twenty patients were men and the median age was 39 (25-64) (Table 1). Thirteen patients (52%) had cirrhosis and 20 patients (80%) were positive for HBeAg. The median length of treatment with ADV-ETV combination therapy was 12 (6-24) months.
Table 1.
Baseline characteristics of nucleoside-refractory patients with chronic hepatitis B virus (HBV) infection (n=25)
*The patient who had been referred due to a high viral load during lamivudine (LAM) plus adefovir dipivoxil (ADV) combination treatment and had previously had a LAM-resistant mutation.
HBeAg, hepatitis B e-antigen; AST, aspartate aminotransferase; ALT, alanine aminotrasferase; HBV, hepatitis B virus; ETV, entecavir; ADV, adefovir dipivoxil; LMV, lamivudine; NAs, nucleos(t)ide analogues.
All patients had previously failed to respond to LAM monotherapy. All patients had taken at least two NAs. Fourteen patients (56%) had failed to respond to two NAs (LAM and either ADV or ETV) and 11 (54%) had failed to respond to three NAs (LAM, ADV and ETV).
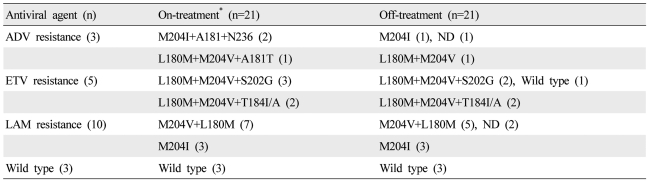
Profiles of genotypic resistance at baseline and after the study time point
On-treatment
At the start of ADV-ETV combination therapy, 12 patients (48%) had documented resistance mutations to LAM only, four (16%) to LAM and ADV (rtA181V/T and/or rtN236T), and six (24%) to LAM and ETV (rtT184 or rtS202). Mutations to NAs were not documented in three patients (12%) They had had VBT during the previous LAM treatment period and had a persistent high viral load during ADV add-on or ETV monotherapy. The HBV DNA levels of the three patients at baseline were 6.4, 5.7 and 4.5 log. All three patients had received LAM followed by ETV monotherapy.
Off-treatment
In the off-treatment time point, four samples were not available. Therefore, the data of 21 patients who had both samples at on- and off-treatment time points were analyzed. The HBV DNA was not detectable in three samples at off-treatment time point.
ADV resistance (three patients): Three patients had ADV and LAM resistance at the on-treatment time point. ADV resistance had disappeared at the off-treatment time point in three patients including one patient whose HBV DNA was undetectable.
ETV resistance (five patients): Four of five patients with both ETV resistance on-treatment had the same profile at the off-treatment time point. One patient had a changed resistance profile after the treatment, namely, ETV resistance was lost and only LAM resistance remained.
LAM resistance (ten patients): The LAM resistance was maintained during the treatment. But two patients who had YVDD mutant at the on-treatment time point achieved complete virological response.
Wild type (three patients): The three patients with wild type at on-treatment did not acquire any genotypic resistance during the treatment period. The HBV DNA levels of these three patients decreased to ≤3.3 log (Table 2).
Table 2.
Genotypic resistance profiles at the on-treatment and off-treatment time points
*The number of all patients were 25, but the blood samples of the off-treatment time point in four patients were not available.
ND, not detectable; ADV, adefovir dipivoxil; ETV, entecavir; LAM, lamivudine.
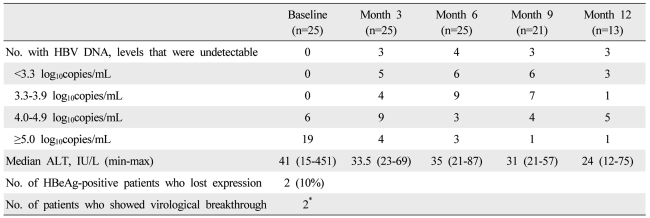
Virological and biochemical responses to combination therapy
From the start to 6 months of treatment, the proportion of patients with fewer than 4 log rose from 0 to 76%. However, the CVR at 9 months of treatment dropped 54%. The median pre-treatment HBV DNA level of all patients was 5.7 log (4.5-9.0). Fourteen patients had elevated alanine aminotransferase (ALT) at baseline (Table 3). After 3 and 6 months of ADV-ETV combination therapy, respectively, three (12%) and four (16%) of the patients had undetectable HBV DNA levels, that is, complete virological response as determined by a PCR assay whose minimum detection limit was <70 copies/mL.
Table 3.
Virological, serological, and biochemical responses to combination therapy after 3, 6, 9, and 12 months of 25 nucleoside-resistant patients with chronic HBV infection
*The virological breakthrough in two patients occurred at 6 and 15 months, respectively.
HBV, hepatitis B virus; HBeAg, hepatitis B e antigen; ALT, alanine aminotransferase.
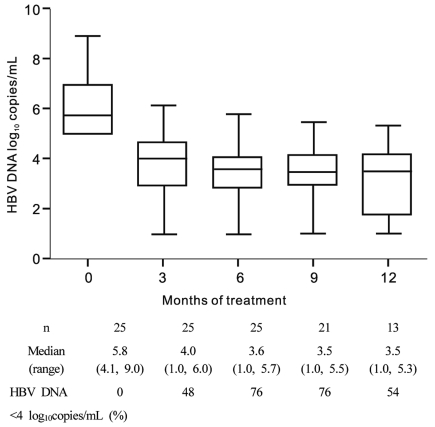
Nineteen of the 25 patients (76%) had an IVR (Table 3) (Fig. 1). Of the 20 patients who were HBeAg-positive at baseline, two (10%) lost HBeAg during ADV-ETV combination therapy at 10.5 months on average (Table 3).
Figure 1.
Distribution of nucleoside-resistant patients with chronic hepatitis B virus (HBV) infection relative to their HBV DNA levels. All patients received combination treatment with entecavir (ETV) and adefovir (ADV). The log HBV DNA levels indicated on the y axis reflect all observations within that exponent. HBV DNA levels of <70 copies/mL could not be detected by polymerase chain reaction analysis. The proportion of patients with a high viral load dropped from 76% at baseline to 5% after 9 months of treatment.
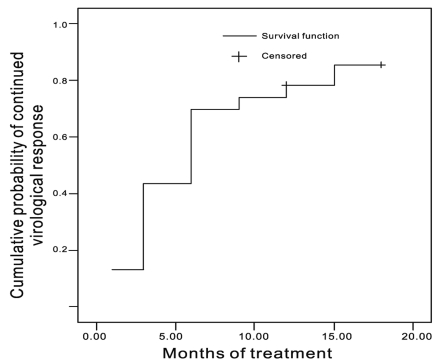
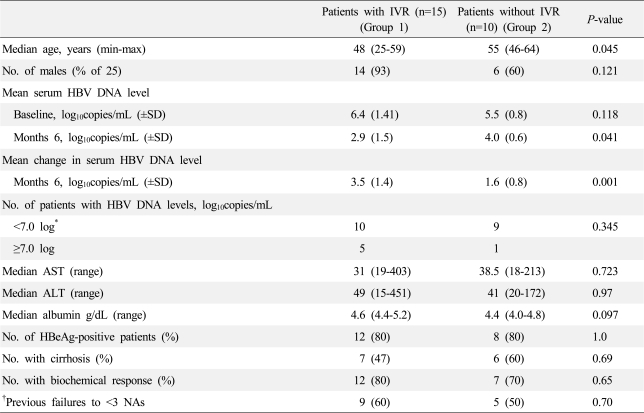
The graph of the cumulative CVR was steep during the early treatment period. After 6 months of treatment, the slope of the curve was gentle (Fig. 2). The patients with and without IVR did not differ significantly in any of the clinical variables, although the patients with IVR to have a lower baseline HBV DNA level (P=0.052) (Table 4).
Figure 2.
Cumulative probability of achieving a continued virological response (CVR), defined as HBV DNA < 4 log10copies/mL after 6 months of ETV+ADV combination treatment. The cumulative probabilities of CVR at months 1, 3, 6, 9, 12, and 15 were 26%, 43%, 70%, 74%, 78%, and 85%, respectively; there were 22, 17, 13, 7, 6, and 3 patients at these time points.
Table 4.
Baseline factors associated with an initial virological response (IVR)
*This standard was taken from Cho et al14; † Number of patients who were exposed to less than 3 NAs.
HBV DNA, hepatitis B virus DNA; IVR, initial virological response; NAs, nucleos(t)ide analogues; AST, aspartate transaminase; ALT, alanine transaminase; HBeAg, hepatitis B e-antigen.
DISCUSSION
Combination therapy was moderately efficacious for those refractory patients with CHB who participated in the study. The IVR rate was 76%. Moreover, the proportion of patients who had high viral loads (≥5.0 log) dropped from 76% at baseline to 5% after 6 months of treatment. Two patients (10%) lost HBeAg, and the biochemical response rate after the first 6 months of treatment was 76%.
With regard to the IVR in this study, most of the patients who had high viral loads at baseline developed intermediate or low viral loads after combination therapy. While an IVR of 76% may not appear to be particularly high, it can actually be considered to be a good response when one realizes that these patients are multi-drug resistant patients with CHB who previously responded poorly to monotherapies with all other oral NAs.
CVR was defined as the HBV DNA levels below 4 log after the 6 month time point. The theoretical background of this definition was originated from the previous studies. Lim et al defined the virological response as the same level.11 The patients exposed to less NAs showed better treatment response. Lai et al12 suggested that HBV DNA suppression should continue until the level decreases to less than 104 copies/mL to prevent the development of complication.
The CVR dropped from 76% at 6 months to 54% at 9 months (Fig. 1). This is likely to be due to a blip phenomenon: blips can be defined as small fluctuations in HBV DNA levels that do not represent. Another reason was that the four patients with a good response dropped out of the study after 9 months because of the financial burden of the drugs. Actually, their HBV DNA levels at 6 months were 3.2, 3.0, 2.0 and 1.0 log, respectively.
The cumulative probability of achieving CVR was shown by Figure 2. Kaplan-Meier analysis could be applied only if rebound HBV DNA levels after CVR were not observed in any of the patients during the observation period. Therefore, two patients with breakthroughs at 6 and 15 months, respectively, were excluded for Kaplan Meier analysis. We strongly suspect that these two patients showed poor compliance at these time points.
The sequential use of NAs monotherapies increases the risk of selection of multi-drug resistant strains.13 As a result, the development of multi-drug resistance to LAM, ADV and ETV is becoming a significant problem in Korea.14 Combination therapy with ETV and tenofovir has been recommended for the treatment of patients who are resistant to LAM and ADV.15 However, since tenofovir is currently not available in Korea, we speculated that ADV-ETV combination therapy may be a good alternative for patients with multi-drug resistance. However, Korean government insurance only permits the use of one antiviral agent in principle, which means that combination therapy places a considerable financial burden on patients in Korea. Drug adherence should be approached as a social problem rather than as a patient's problem.
Baseline clinical prognostic factors that could predict IVR or CVR could not be identified. However, if the patients were divided into two groups according to whether they exhibited a decrease in HBV DNA by more than 2 log after 6 months of treatment, only age differed significantly between the two groups upon univariate analysis (data not shown). Multi-variate analysis was performed for four significant factors (P-value <0.1 in univariate analysis). However, none of these factors were proven to be prognostic factors for IVR upon multi-variate analysis (data not shown). Another study showed that patients who were refractory to ADV and who achieved IVR with ETV monotherapy had higher baseline ALT and AST levels than those who did not achieve IVR.14 A recent study also showed that low pre-treatment HBV DNA levels and previous exposure to fewer NAs were associated with a virological response.11
The loss of HBeAg or a biochemical response during the early treatment period could not be analyzed because too few of the patients had HBeAg (n=20) or high ALT levels (n=16) at baseline. One patient exhibited a biochemical VBT after 15 months of treatment. This patient confessed that he had sometimes failed to take his medication around 15 months after treatment. A new report about medication compliance that was published recently found that nine of 10 patients who experienced an initial VBT, but did not have confirmed genotypic resistance, had undetectable HBV DNA levels at the most recent follow-up, which was on average 7 months after the initial VBT. These data suggest that medication noncompliance can be a common cause of VBT.16
With regard to the genotypic resistance data of the present study, the genotypic resistance profile at the on-treatment time point was compared to that at the off-treatment time point. Mutations associated with LAM and ETV resistance were maintained in four of five patients, but ADV resistance had disappeared in three patients by the off-treatment time point. We speculate that ETV can somehow control ADV-resistant viruses, but ADV cannot control the virus with ETV-and LAM-resistant viruses. Indeed, Reijnders et al17 reported that viruses with ADV-resistant mutations are expected to remain sensitive to ETV. Moreover, in vitro study reported that viruses with the ADV-resistance rtN236T mutation remains sensitive to ETV, albeit with a <3-fold change in IC50, while viruses with the ADV-resistance rt181V/T mutations remain completely sensitive to ETV.18,19
The limitations of the present study were, first, that the follow-up period was short. Second, drug adherence in the patients was not checked. The three patients who had taken ETV showed an inadequate response to the ADV-ETV combination therapy and we suspect poor drug adherence in these patients. Third, four patients dropped out because of the economic burden of the drug regimen. Two patients completed 6 months of treatment, and the other two patients completed 9 months of treatment. Finally, the present study was retrospective.
In conclusion, patients who experienced ADV or ETV resistance after becoming refractory to LAM showed a good IVR in response to ADV-ETV combination therapy. This combination therapy appeared to be more effective for ADV-resistance mutations than ETV resistance mutations. This combination regimen is thus a good alternative or bridge therapy for patients with multi-drug resistance until tenofovir become available. Further studies with larger number of patients are needed to determine the exact efficacy of this regimen and the factors that favor a good IVR.
Acknowledgements
This study was supported by a grant from the National R&D Program for Cancer Control, Ministry of Health and Welfare, Republic of Korea (1120330).
Abbreviations
- ADV
adefovir dipivoxil
- ALT
alanine aminotransferase
- anti-HBe
anti-hepatitis B e-antigen
- AST
aspartate aminotransferase
- CHB
chronic hepatitis B
- CVR
continued virological response
- ETV
entecavir
- HBeAg
hepatitis B e-antigen
- HBsAg
hepatitis B surface antigen
- HBV
hepatitis B virus
- IVR
initial virological response
- LAM
lamivudine
- Log
log10HBV DNA copies/mL
- NAs
nucleos(t)ide analogues
- ND
not detectable
- TDF
tenofovir disoproxil fumarate
- VBT
virologic breakthrough
Appendix Table 1
Baseline factors associated with an IVR (defined as a 2 log decrease from the baseline)
*This standard was taken from Cho et al14; † Number of patients who were exposed to less than 3 NAs.
HBV DNA, hepatitis B virus DNA; IVR, initial virological response; NAs, nucleos(t)ide analogues; AST, aspartate transaminase; ALT, alanine transaminase; HBeAg, hepatitis B e-antigen.
References
- 1.European Association For The Study Of The Liver. EASL Clinical Practice Guidelines: management of chronic hepatitis B. J Hepatol. 2009;50:227–242. doi: 10.1016/j.jhep.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50:661–662. doi: 10.1002/hep.23190. [DOI] [PubMed] [Google Scholar]
- 3.Ganem D, Prince AM. Hepatitis B virus infection-natural history and clinical consequences. N Engl J Med. 2004;350:1118–1129. doi: 10.1056/NEJMra031087. [DOI] [PubMed] [Google Scholar]
- 4.Wright TL, Lau JY. Clinical aspects of hepatitis B virus infection. Lancet. 1993;342:1340–1344. doi: 10.1016/0140-6736(93)92250-w. [DOI] [PubMed] [Google Scholar]
- 5.Hansen BE, Buster EH, Steyerberg EW, Lesaffre E, Janssen HL. Prediction of the response to peginterferon-alfa in patients with HBeAg positive chronic hepatitis B using decline of HBV DNA during treatment. J Med Virol. 2010;82:1135–1142. doi: 10.1002/jmv.21778. [DOI] [PubMed] [Google Scholar]
- 6.Tenney DJ, Rose RE, Baldick CJ, Pokornowski KA, Eggers BJ, Fang J, et al. Long-term monitoring shows hepatitis B virus resistance to entecavir in nucleoside-naïve patients is rare through 5 years of therapy. Hepatology. 2009;49:1503–1514. doi: 10.1002/hep.22841. [DOI] [PubMed] [Google Scholar]
- 7.Fung SK, Chae HB, Fontana RJ, Conjeevaram H, Marrero J, Oberhelman K, et al. Virologic response and resistance to adefovir in patients with chronic hepatitis B. J Hepatol. 2006;44:283–290. doi: 10.1016/j.jhep.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 8.Cho JH, Yoon KH, Lee KE, Park DS, Lee YJ, Moon HB, et al. Distribution of hepatitis B virus genotypes in Korea. Korean J Hepatol. 2009;15:140–147. doi: 10.3350/kjhep.2009.15.2.140. [DOI] [PubMed] [Google Scholar]
- 9.Bae SH, Yoon SK, Jang JW, Kim CW, Nam SW, Choi JY, et al. Hepatitis B virus genotype C prevails among chronic carriers of the virus in Korea. J Korean Med Sci. 2005;20:816–820. doi: 10.3346/jkms.2005.20.5.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee JM, Ahn SH, Chang HY, Shin JE, Kim DY, Sim MK, et al. Reappraisal of HBV genotypes and clinical significance in Koreans using MALDI-TOF mass spectrometry. Korean J Hepatol. 2004;10:260–270. [PubMed] [Google Scholar]
- 11.Lim YS, Lee TH, Heo NY, Shim JH, Lee HC, Suh DJ. Entecavir plus adefovir combination treatment for chronic hepatitis B patients after failure of nucleoside/nucleotide analogues. Antivir Ther. 2012;17:53–60. doi: 10.3851/IMP1914. [DOI] [PubMed] [Google Scholar]
- 12.Lai CL, Yuen MF. The natural history and treatment of chronic hepatitis B: a critical evaluation of standard treatment criteria and end points. Ann Intern Med. 2007;147:58–61. doi: 10.7326/0003-4819-147-1-200707030-00010. [DOI] [PubMed] [Google Scholar]
- 13.Locarnini S, Hatzakis A, Heathcote J, Keeffe EB, Liang TJ, Mutimer D, et al. Management of antiviral resistance in patients with chronic hepatitis B. Antivir Ther. 2004;9:679–693. [PubMed] [Google Scholar]
- 14.Cho SW, Koh KH, Cheong JY, Lee MH, Hong SP, Yoo WD, et al. Low efficacy of entecavir therapy in adefovir-refractory hepatitis B patients with prior lamivudine resistance. J Viral Hepat. 2010;17:171–177. doi: 10.1111/j.1365-2893.2009.01161.x. [DOI] [PubMed] [Google Scholar]
- 15.Lok AS, Zoulim F, Locarnini S, Bartholomeusz A, Ghany MG, Pawlotsky J, et al. Antiviral drug-resistant HBV: standardization of nomenclature and assays and recommendations for management. Hepatology. 2007;46:254–265. doi: 10.1002/hep.21698. [DOI] [PubMed] [Google Scholar]
- 16.Lok AS, Wu PC, Lai CL, Lau JY, Leung EK, Wong LS, et al. A controlled trial of interferon with or without prednisone priming for chronic hepatitis B. Gastroenterology. 1992;102:2091–2097. doi: 10.1016/0016-5085(92)90337-x. [DOI] [PubMed] [Google Scholar]
- 17.Reijnders JG, Pas SD, Schutten M, de Man RA, Janssen HL. Entecavir shows limited efficacy in HBeAg-positive hepatitis B patients with a partial virologic response to adefovir therapy. J Hepatol. 2009;50:674–683. doi: 10.1016/j.jhep.2008.10.033. [DOI] [PubMed] [Google Scholar]
- 18.Qi X, Xiong S, Yang H, Miller M, Delaney WE., 4th In vitro susceptibility of adefovir-associated hepatitis B virus polymerase mutations to other antiviral agents. Antivir Ther. 2007;12:355–362. [PubMed] [Google Scholar]
- 19.Villet S, Pichoud C, Billioud G, Barraud L, Durantel S, Trépo C, et al. Impact of hepatitis B virus rtA181V/T mutants on hepatitis B treatment failure. J Hepatol. 2008;48:747–755. doi: 10.1016/j.jhep.2008.01.027. [DOI] [PubMed] [Google Scholar]