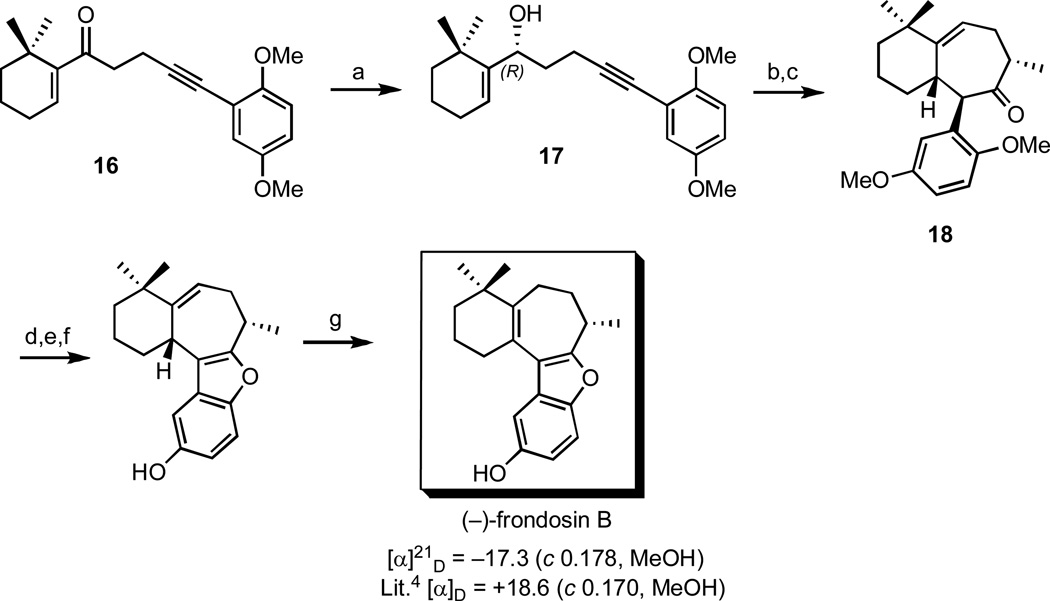
Scheme 5.
Total synthesis of (–)-frondosin B.
Reagents and conditions: (a) catechol borane, (–)-CBS reagent, toluene, −78 °C, 72h, 68%, 98% ee, (b) cat. MeLi, 210 °C (µwave), 1h, 79% (c) i. LHMDS, THF, −78 °C, ii. MeI, −60 °C, 85%, 97% ee, (d) CAN, MeCN/H2O, rt, 93% (e) i. H2, Pd/C, 5 min, (f) BF3•OEt2, DCM, 0 °C, 2% (2 steps), (g) cat. TsOH, benzene, reflux 5h, 68%.