Abstract
The age-prospective memory (PM) paradox asserts that, despite evidence of age-associated PM deficits on laboratory tasks, older adults perform comparably to (or better than) young adults on naturalistic PM tasks. This study examined the age-PM paradox in older HIV-infected individuals, who represent a growing epidemic and may be at heightened risk for adverse neurocognitive and everyday functioning outcomes. Participants included 88 older (50+ years) and 53 younger (≤40 years) HIV-infected individuals as well as 54 older and 59 younger seronegative adults who completed both laboratory and naturalistic time-based PM tasks. Similar interactions were observed in both the seropositive and the seronegative samples, such that the older participants demonstrated significantly lower laboratory-based PM than the younger groups, but not on the naturalistic PM trial. Secondary analyses within the HIV+ sample revealed that naturalistic task success was indirectly associated with greater self-reported use of PM-based and external compensatory strategies in the daily lives of older, but not younger, HIV+ adults. Study findings suggest that, although older HIV-infected adults exhibit moderate PM deficits on laboratory measures versus their younger counterparts, such impairments are paradoxically not evident on ecologically relevant naturalistic PM activities in daily life, perhaps related to effective utilization compensatory strategies.
Keywords: Episodic memory, Aging, Neuropsychological assessment, AIDS dementia complex, Cognition
It has been reliably demonstrated that the normal pattern of cognitive declines associated with aging may include decrements in prospective memory (PM), or the ability to remember to execute an intention in the future (Einstein & McDaniel, 1990). Moderate deficits are seen across both time- and event-based PM tasks in the laboratory (Henry, MacLeod, Phillips, & Crawford, 2004) and tend to be exacerbated as executive demands increase (McDaniel & Einstein, 2000), for example with nonfocal versus focal cues (Kliegel, Martin, McDaniel, & Phillips, 2007). Yet a surprising contradiction exists such that, despite strong evidence of laboratory-based PM declines, older adults tend to perform better than (or at least comparably to) younger counterparts on naturalistic PM tasks meant to mimic everyday life (Henry et al., 2004). For instance, older adults are often superior to young adults at tasks such as remembering to phone the examiner at a later date (e.g., Maylor, 1990) and/or mail a postcard back to the laboratory (e.g., Patton & Meit, 1993). Dubbed “the age-PM paradox” by Rendell and Thomson (1999), these puzzling findings first emerged in the literature nearly 20 years ago (e.g., Rendell & Thomson, 1993) and corroborate long-standing clinical impressions that older adults are often more reliable in complying with their healthcare in everyday life (e.g., attending appointments, adhering to medications). Numerous theories have been offered, with mixed empirical support, to explain how older adults are able to overcome declines in PM when outside the laboratory, including age-related differences in personality factors (e.g., increased conscientiousness; Patton & Meit, 1993; Rendell & Craik, 2000), increased lifestyle structure and reduced busyness (Schnitzspahn, Ihle, Henry, Rendell, & Kliegel, 2011; cf. Bailey, Henry, Rendell, Phillips, & Kliegel, 2010), and greater use of external aids and other mnemonic strategies (Maylor, 1990; Moscovitch, 1982; cf. Rendell & Thomson, 1993, 1999).
Although a significant body of work has examined the impact of aging on laboratory-based and naturalistic PM separately, remarkably few studies have directly examined the paradox in a single cohort (i.e., using within-subjects designs). The first study to explore the paradox in this preferred fashion was conducted by Rendell and Thomson (1999), who observed that younger adults (mean age = 20.2 years) performed better than older adults (young-old mean age = 65.0 years; old-old mean age = 83.2 years) on two time-based PM tasks administered in the laboratory; however, both groups of older adults were more likely to complete the naturalistic PM task, which involved keying a specific sequence onto an electronic organizer at four prespecified times during normal daily activities. In a subsequent study, Bailey et al. (2010) demonstrated that older adults were more likely to remember to respond to a set of questions via personal digital assistants (PDAs) outside the laboratory, but younger adults performed better on the experimenter-controlled laboratory-based PM task that was embedded within a naturalistic PDA questionnaire. Older adults in this study were comparably active in their level and type of ongoing activities during the naturalistic PM task (e.g., shopping), suggesting that their superior performance may not be due to decreased busyness, but perhaps instead to the attentional demands and predictability associated with such a routine. Most recently, Schnitzspahn and colleagues (2011) examined performance on similar laboratory and naturalistic tasks that required the participant to either press a key (during laboratory task) or text a simple message (during naturalistic task) at specified times. Using this within-subjects design, the authors observed the expected age-PM paradox and also noted that older adults reported higher motivation to complete the naturalistic task, had greater metacognitive awareness regarding their naturalistic PM abilities, and reported lower levels of everyday stress.
Yet our understanding of the age-PM paradox in clinical samples thus far is quite limited (e.g., Raskin et al., 2011; Will et al., 2009), which is somewhat surprising given the growing evidence of PM deficits in a range of medical, psychiatric, and neurological populations (see Kliegel, Jäger, Altgassen, & Shum, 2008) and the relevance of such deficits to successful independent living (e.g., Woods, Iudicello, et al., 2008). For example, this phenomenon and its mechanisms may be particularly relevant in the context of HIV infection, where older adults now represent a substantial proportion of the total epidemic in the Western world, largely due to the success of antiretroviral therapies (Centers for Disease Control and Prevention, 2007). Increasing evidence suggests that older adults with HIV may have more rapid disease progression (e.g., Goetz, Boscardin, Wiley, & Alkasspooles, 2001) and increased morbidity (Perez & Moore, 2003). Furthermore, there may be a greater likelihood of HIV-associated neurocognitive disorders in older HIV-infected adults (Valcour et al., 2004), including deficits in retrospective memory and executive functions (Cherner et al., 2004; Sacktor et al., 2007; cf. Valcour, Paul, Neuhaus, & Shikuma, 2011), both of which contribute to optimal PM performance (Carey, Woods, Rippeth, Heaton, Grant, & The HNRC Group, 2006; Gupta et al., 2010; Kliegel et al., 2008).
In accordance with its preferential pattern of frontostriatal neural injury (Ellis, Calero, & Stockin, 2009), HIV infection is broadly associated with moderate deficits in both time- and event-based PM (Carey et al., 2006; Martin et al., 2007; Zogg et al., 2011) that are characterized by difficulties in self-initiated encoding, monitoring, and retrieval. Moreover, individuals with HIV express more PM complaints in their daily lives (Woods et al., 2007) and may be at risk for deficits on PM naturalistic tasks as compared to their seronegative counterparts (Carey et al., 2006). Speaking to the ecological relevance of these findings, HIV-associated PM deficits in the laboratory are associated with a range of poorer everyday functioning outcomes, including dependence in instrumental activities of daily living (Woods, Iudicello, et al., 2008), medication nonadherence (Contardo, Black, Beauvais, Dieckhaus, & Rosen, 2009; Woods et al., 2009), and unemployment (Woods, Weber, Weisz, Twamley, Grant, & The HNRP Group, 2011). For example, Woods, Iudicello, and colleagues (2008) found that HIV+ individuals who demonstrated impairment on a laboratory-based PM task possessed a fourfold risk of reporting declines in the independent management of their instrumental activities of daily living, an effect that was independent of other cognitive impairments, psychiatric comorbidity, and HIV disease severity.
Despite its demonstrated incremental validity in predicting everyday functioning outcomes in HIV, less is known about the potential impact of age as a moderating factor on this relationship. In other words, it is unclear whether older individuals with HIV are spared from everyday PM failures in the face of significant deficits in the laboratory, as would be predicted by the age-PM paradox, or whether the moderate neurocognitive declines associated with comorbid HIV and aging override the apparent protective factors for naturalistic PM associated with aging in otherwise healthy samples (Barclay et al., 2007). A closer review of the extant neuroAIDS literature nevertheless provides several hints that the age-PM paradox may also exist in HIV infection. With regard to laboratory PM, Woods, Dawson, Weber, Grant, and the HNRC Group (2010) observed additive effects of age and HIV infection on event-based PM, which were exacerbated especially when executive demands were increased; in fact, they noted a significant relationship between older age and worse PM performance even within the older HIV-infected cohort. In terms of naturalistic tasks, older adults are more adherent to their antiretroviral medications than younger HIV+ adults (Hinkin et al., 2004), an activity that conceptually maps onto PM (Park & Kidder, 1996; Zogg, Woods, Sauceda, Wiebe, & Simoni, 2011, for a review). More recently, Zogg and colleagues (2010) reported no significant age effects on a naturalistic PM telephone task in a large, but predominately middle-aged HIV-infected cohort. Thus, it appears that, despite evidence of age-associated PM deficits in the laboratory, which are risk factors for poorer everyday functioning (Barclay et al., 2007), older HIV-infected adults tend to perform adequately on naturalistic PM tasks. Yet no studies have directly examined the age-PM paradox (i.e., both laboratory and naturalistic PM tasks) in older and younger HIV-infected adults.
As such, the primary aim of the following study was to examine whether the age-PM paradox exists within an HIV+ sample using a within-subjects design. Secondarily, we sought to explore whether naturalistic PM task success was indirectly associated with greater self-reported use of compensatory strategies in the daily lives of HIV-infected persons. This question is especially important to extend from the healthy aging literature to HIV (and other neuromedical disorders), given that there are likely more severe PM deficits, intensified health-related everyday demands (e.g., complex medication regimens, doctors appointments), and practice with compensatory strategies (e.g., pillboxes, cell phone reminders).
METHOD
Participants
This study was approved by the institution’s human research protections program. Participants included 141 HIV-infected and 113 HIV-seronegative individuals recruited via flyer or newspaper advertisement from the San Diego community and local HIV clinics. Exclusion criteria were severe psychiatric illness (e.g., schizophrenia), neurological disease (e.g., seizure disorders, stroke, closed head injury with loss of consciousness for more than 15 min, and central nervous system neoplasms or opportunistic infections), verbal IQ scores <70 (based on Wechsler Test of Adult Reading, WTAR; Psychological Corporation, 2001), a diagnosis of substance dependence within 1 month prior to the baseline evaluation, and a urine toxicology screen positive for illicit drugs (excluding marijuana) on the day of testing. The primary inclusion criterion across both serostatus groups was age ≥ 50 (“older”) or ≤ 40 (“younger”) years. Although this grouping resulted in older samples that were younger than is typically considered in geropsychology, it is nevertheless consistent with the HIV epidemic (e.g., Centers for Disease Control and Prevention, 2007) and National Institutes of Health (NIH) recommendations for aging neuroAIDS research (Stoff, 2004). This design yielded four groups defined by age and HIV serostatus: older HIV+ (n = 88), younger HIV+ (n = 53), older HIV− (n = 54), and younger HIV− (n = 59). All four groups were well matched on education and prevalence of lifetime substance dependence, and participants in the older and younger groups were comparable in age across serostatus (ps > .10). However, Tables 1 and 2 show that the study groups were not comparable on a number of demographic, psychiatric, and medical variables, many of which are representative of age- and HIV-associated trends (e.g., increased prevalence of hypercholesterolemia in older groups, higher percentage of men in HIV+ groups).
TABLE 1.
Demographics and psychiatric characteristics of the study samples
| Variable | Young
|
Old
|
p | Pairwise comparisons | ||
|---|---|---|---|---|---|---|
| HIV− (n = 59) | HIV+ (n = 53) | HIV− (n = 54) | HIV+ (n = 88) | |||
| Demographic characteristics | ||||||
| Age (years) | 30.2 (6.0) | 31.9 (5.2) | 56.1 (5.0) | 55.9 (6.0) | <.001 | O+,O− > Y−,Y+ |
| Education (years) | 14.3 (2.4) | 13.3 (2.6) | 14.4 (2.4) | 14.0 (2.4) | .131 | — |
| Sex (% men) | 61.0 | 83.0 | 64.8 | 82.0 | .006 | O+,Y+ > Y−,O− |
| Ethnicity (% Caucasian) | 47.5 | 39.6 | 66.7 | 69.3 | <.001 | O+,O− > Y+,Y− |
| Psychiatric characteristics | ||||||
| Substance dependencea (%) | 40.7 | 49.1 | 51.9 | 52.3 | .535 | — |
| Major depressive disordera (%) | 30.5 | 43.4 | 42.6 | 59.1 | .007 | O+ > Y− |
| Generalized anxiety disordera (%) | 1.7 | 7.6 | 5.6 | 18.2 | .004 | O+ > O−,Y− |
| Profile of Mood States (total)b | 38 (23, 60) | 47 (31, 67.5) | 40.5 (24, 68.8) | 50 (31, 70) | .124 | — |
Note. Y− = younger HIV seronegative; O− = older HIV seronegative; Y+ = younger HIV seropositive; O+ = older HIV seropositive.
Denotes any lifetime diagnosis.
Data represent medians with interquartile ranges in parentheses.
TABLE 2.
HIV disease and medical characteristics of the study samples
| Young
|
Old
|
p | Pairwise comparisons | |||
|---|---|---|---|---|---|---|
| HIV− (n = 59) | HIV+ (n = 53) | HIV− (n = 54) | HIV+ (n = 88) | |||
| Hepatitis C (%) | 3 | 4 | 17 | 34 | <.001 | O+ > O− > Y+, Y− |
| Hypertension (%) | 2 | 9 | 22 | 30 | <.001 | O+ > Y+, Y− |
| Hypercholesterolemia (%) | 0 | 2 | 15 | 19 | <.001 | O+, O− > Y+, Y− |
| Diabetes mellitus (%) | 0 | 2 | 7 | 10 | .031 | O+ > Y− |
| HIV disease characteristics | ||||||
| HIV duration (years)a | — | 6.9 (5.2) | — | 15.3 (7.4) | <.001 | O+ > Y+ |
| AIDS (%) | — | 25 | — | 64 | <.001 | O+ > Y+ |
| cART (%) | — | 70 | — | 81 | .222 | — |
| Nadir CD4b (cells/μl) | — | 265 (204, 356) | — | 123 (40, 300) | <.001 | O+ < Y+ |
| Current CD4b (cells/μl) | — | 559 (404, 840) | — | 512 (308, 738) | .182 | — |
| Detectable plasma HIV RNA (%) | — | 40 | — | 24 | .058 | — |
| Detectable CSF HIV RNAc (%) | — | 28 | — | 16 | .176 | — |
Note. Y− = younger HIV seronegative; O− = older HIV seronegative; Y+ = younger HIV seropositive; O+ = older HIV seropositive; CD4 = cluster of differentiation 4; CSF = cerebrospinal fluid; cART = combination antiretroviral therapy.
Data represent means with standard deviations in parentheses.
Data represent medians with interquartile ranges in parentheses.
O+, n = 56; Y+, n = 36.
Materials and procedure
After providing written informed consent, participants completed a comprehensive neuropsychological, psychiatric, and medical evaluation, for which they received nominal monetary compensation.
Prospective memory assessment
Laboratory-based PM was assessed using the research version of the Memory for Intentions Screening Test (MIST; for more information, the reader is referred to Raskin, Buckheit, & Sherrod, 2010, and Woods, Moran, et al., 2008). The MIST is a 30-min test during which participants are asked to perform eight examiner prescribed intentions, while completing a series of word search puzzles that serve as ongoing tasks. Four time-based trials are given such that a participant is asked to perform a task after either 2 (i.e., “In 2 minutes, ask me what time this session ends today” and “In 2 minutes, tell me two things you forgot to do this past week”) or 15 min (i.e., “In 15 minutes, tell me that it’s time to take a break” and “In 15 minutes, write down the number of medications you are taking on your paper”), counterbalanced between verbal and action responses (see previous example, respectively). Of note, participants are only allowed to use a wall clock situated behind them in order to check the time; external aids, such as writing notes or using a watch, were prohibited from use during this task. The time-based scale has a range of 0–8, such that fully correct trials receive 2 points (i.e., correct PM and retrospective, RM, components), trials with a missing PM (e.g., action performed at the incorrect time) or RM (e.g., wrong action performed at the correct time) component received 1 point, and omission errors received 0 points. As such, the MIST allows for error type coding as follows: (a) no response (i.e., omission errors); (b) loss of time (i.e., performing the correct response at the wrong time; ≥15% of the target time); (c) task substitution (e.g., perseverations or intrusions, such as replacing a verbal response with an action or vice versa); and (d) loss of content (e.g., acknowledging that a response is required, but failing to recall the particulars). After completing the primary task, participants were administered an eight-item, three-choice recognition trial (range = 0–8).
Prior to beginning their neuropsychological assessment, participants were given a naturalistic PM task instruction, which was also adapted from the research version of the MIST (Raskin et al., 2010; Woods, Moran, et al., 2008). For this task, each participant was instructed to telephone the examiner at a specific time the following day and leave a voicemail message specifying the number of hours slept the previous night (Carey et al., 2006; Zogg et al., 2010). Although not explicitly mentioned or encouraged by the examiner, the use of mnemonic strategies such as electronic calendars was permitted. Participants were given full credit for completing the task if the correct message was left for the examiner the next day at the target time. Conversely, performance was scored as “incomplete” if the participant did not leave any message, did so at an incorrect time (≥15% of the target time; i.e., ±3 hours and 35 minutes), or left a message without specifying the number of hours slept. Prior research supports the reliability (Woods, Moran, et al., 2008) and construct validity (Woods et al., 2009; Woods, Iudicello, et al., 2008, Gupta et al., 2010) of the MIST, including the time-based scale and 24-hour trial (Carey et al., 2006; Zogg et al., 2010) in HIV infection.
As an indicator of self-reported PM complaints, participants completed the Prospective Memory Scale from the Prospective and Retrospective Memory Questionnaire (PRMQ; Smith, Della Sala, Logie, & Maylor, 2000), on which individuals noted the frequency with which they experience failures of PM in their daily lives. Additionally, participants were administered the Prospective Memory for Medications Questionnaire (PMMQ; Gould, McDonald-Miszczak, & King, 1997) as a measure of mnemonic strategy use in daily life. The PMMQ is a 28-item self-report questionnaire that yields four subscales corresponding to use of internal (e.g., “repeat the instructions in my head”), external (e.g., “write a note”), PM-based (e.g., “ask someone to remind me when I need to take my medications”), and RM-based (e.g., “concentrate while reading the medication instructions”) strategies associated with remembering to take medications. Items are rated on a Likert-type scale based on frequency of strategy use in the last month, with higher scores indicating greater frequency (e.g., 0 = “never use this strategy,” 4 = “always use this strategy”).
Psychiatric assessment
Lifetime and current diagnoses of major depressive disorder, generalized anxiety disorder, and substance use disorders were generated from structured psychiatric interviews conducted using the Composite International Diagnostic Interview (Version 2.1; World Health Organization, 1998) according to Diagnostic and Statistical Manual of Mental Disorders–Fourth Edition criteria (American Psychiatric Association, 1994). Participants also completed the Profile of Mood States (POMS; McNair, Lorr, & Droppleman, 1981), which measures current affective distress in the areas of tension/anxiety, depression/dejection, anger/hostility, vigor/activity, fatigue/inertia, and cognitive/confusion. The POMS also provides a total mood disturbance score, on which higher scores signal greater distress.
RESULTS
Age-PM paradox
The present study examined the age-PM paradox using a mixed model analysis of variance (ANOVA). The two between-subjects factors were age group and HIV serostatus. The within-subjects factor was PM task type (i.e., laboratory based or naturalistic).
Between-subject factors
Analyses revealed significant main effects of both between-subjects factors (i.e., HIV status and age group), such that overall PM performance was worse in the HIV-infected, F(1, 250) = 5.10, p = .025, η2 = .020, and older subgroups, F(1, 250) = 20.47, p < .001, η2 = .076. The Age × HIV interaction term was not significant, F(1, 250) = 1.14, p = .288, η2 = .005.
Age Group × Task interaction
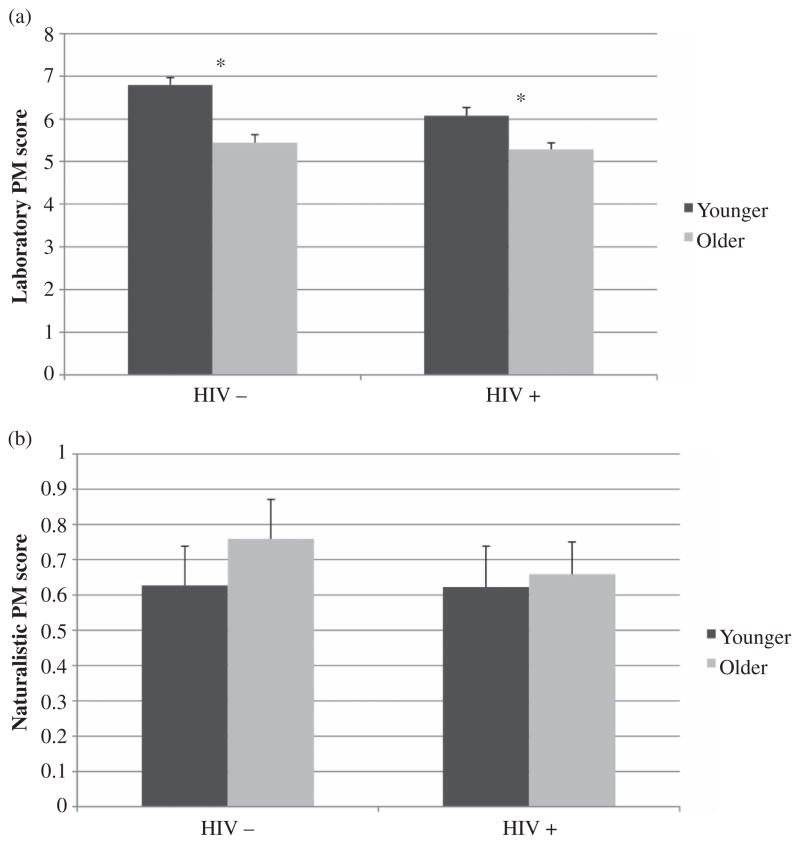
In regards to performance between task types (i.e., within-subjects factor), the interaction between age group and task type was significant, F(1, 250) = 33.47, p < .001, η2 = .118. Planned follow-up analyses indicated that older individuals performed worse on the laboratory task (i.e., MIST time subscale) than did their younger counterparts, t(240.15) = −6.23, p < .001, d = 0.86, but age groups did not differ on the naturalistic task (i.e., 24-hour trial), t(238.71) = −0.68, p > .10, d = 0.08.
HIV Status × Task interaction
A trend-level interaction was present between HIV status and task, F(1, 250) = 3.78, p = .053, η2 = .015. Planned post hoc analyses revealed a similar pattern, whereby the discrepancy between serostatus groups on the MIST time scale was significant and was associated with a medium effect size, t(239.64) = −3.02, p = .003, d = 0.40, but naturalistic performance was comparable across serostatus, t(241.38) = −0.43, p > .10, d = 0.08.
Age Group × HIV Status × Task interaction
Finally, the three-way interaction term (Age × Serostatus × Task Type) was not significant, F(1, 250) = 2.70, η2 = .011, p > .10. Planned post hoc analyses showed that the age group finding on the MIST time scale was significant in the HIV+ cohort, t(112.71) = 3.18, p < .004, d = 0.55. This finding held even after including demographic (i.e., sex, ethnicity) and disease (i.e., nadir cluster of differentiation 4, CD4; AIDS status; duration of infection; hepatitis C infection; hypertension; hypercholesterolemia; diabetes; generalized anxiety disorde diagnosis) variables on which the age groups differed in the statistical model, R2 = .15, F(10, 130) = 2.25, p = .019. A robust age effect was also found on the MIST time scale in the HIV− group, t(109.07) = 5.35, p < .001, d = 1.04; similarly, these effects remained significant even after considering factors on which the age groups differed (i.e., ethnicity, hypercholesterolemia, hepatitis C infection) in the statistical model, R2 = .22, F(3, 109) = 10.52, p < .001. There were no significant age differences on the naturalistic tasks in either serostatus group: HIV+, t(111.65) = −0.25, p > .10, d = 0.05; HIV−, t(110.63) = −0.84, p > .10, d = 0.16 (see Figures 1a and 1b).
Figure 1.
Bar charts display the interaction between age and laboratory (a) and naturalistic (b) prospective memory performance in persons with and without HIV infection. *p < .05.
Alternative analytic approaches
In order to confirm the significance findings of the primary analysis, additional multivariate analyses of variance (MANOVAs) were conducted with potential confounding factors in the model as covariates. These three MANOVAs were determined based on category of variables on which all four groups differed and were clustered into demographic (i.e., sex, ethnicity), psychiatric (i.e., lifetime diagnoses of major depressive disorder [MDD] and generalized anxiety disorder [GAD]), and disease (i.e., hepatitis C, hypertension, hypercholesterolemia, diabetes mellitus) variables. The addition of these confounding factors into the models did not change the primary age-PM paradox results described above (all ps < .05). To increase consistency with other studies in the PM literature, the naturalistic task was also analyzed using a three-group indicator of task success in order to isolate the PM component of the task (i.e., “correct PM and RM component” = call at the correct time with the correct information, “correct PM component” = call at the correct time but with incorrect or absent information, and “missing” = no call). These analyses did not differ from the aforementioned approach and findings.
Compensatory strategies and naturalistic task success
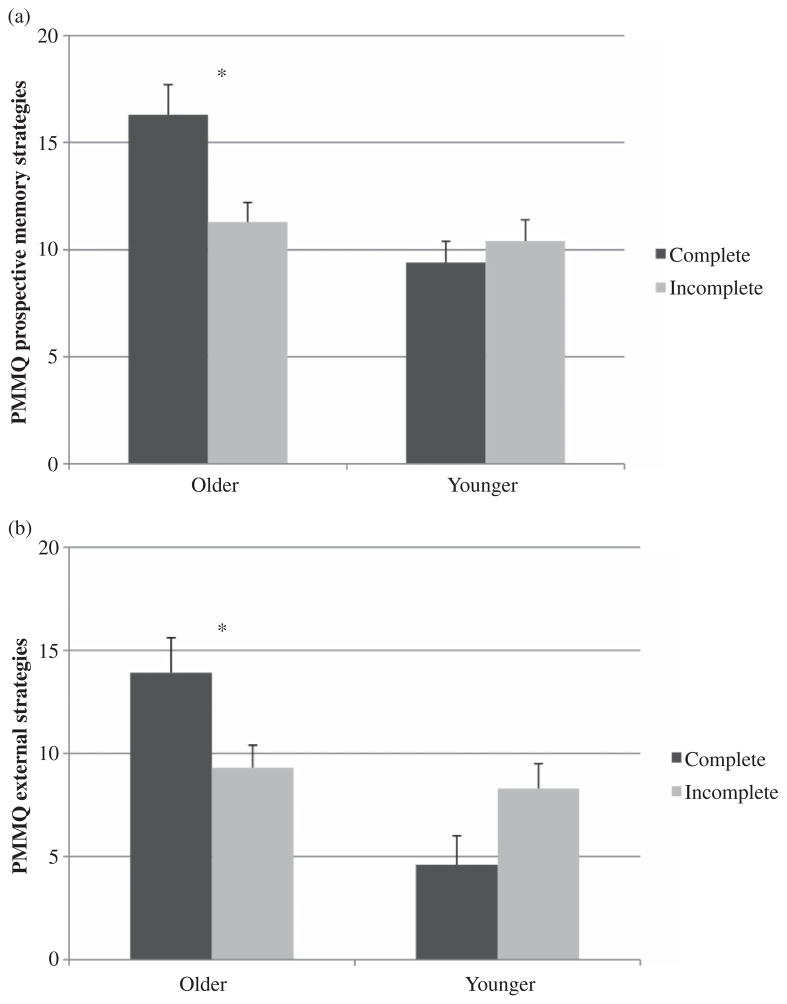
To explore the predictors of naturalistic task success in the HIV+ group, logistic regressions were conducted with age group, PMMQ subscales, and their interactions as predictors of 24-hour trial completion (i.e., correct PM and RM components). For the PMMQ PM subscale, the full model was significant— χ2(3) = 10.90, p = .01—and did not yield significant main effects of either age group or subscale score (both ps > .10) in predicting naturalistic task completion. Similarly, the full model for the PMMQ external strategy subscale was significant— χ2(3) = 8.93, p = .03—with no main effects of age group or subscale score. Importantly, there were interactions between age group and use of both PM-based, χ2(1) = 4.08, p = .043, and external, χ2(1) = 5.38, p = .020, mnemonic strategies on 24-hour task completion but not RM-based—χ2(3) = 5.55, p > .10; interaction term, χ2(1) = 2.51, p > .10—or internal strategies— χ2(3) = 5.99, p > .10; interaction term, χ2(1) = 1.23, p > .10. That is, only older HIV+ participants who successfully completed the naturalistic task reported using more PM-based, t(33.42) = −3.40, p = .002, d = 0.77, and external, t(33.74) = −2.61, p = .013, d = 0.64, mnemonic strategies in their daily lives (see Figures 2a and 2b). (N.B., this secondary analysis was performed only in older individuals who were prescribed medication, n = 85, as the PMMQ only evaluates mnemonic strategy use as it pertains to medication management; relatedly, the primary analyses detailed above remained significant when performed using this combination antiretroviral therapy (cART) restriction criterion in all HIV+ individuals, n = 120.) Potential confounding factors on which the seropositive age groups differed (i.e., ethnicity, hepatitis C status, hypertension, hypercholesterolemia, duration of infection, and AIDS status) were all including both the PM subscale— χ2(9) = 17.21, p = .046—and external subscale— χ2(9) = 16.81, p = .052—logistic regressions, but did not change the significance of the age group by strategy use interaction (all ps < .05). Within these factors, AIDS status was an independent predictor of 24-hour trial completion such that having an AIDS diagnosis was associated with completing the trial across both age groups (ps < .05). Further analyses revealed that there were no other significant predictors or interactions on any of the other demographic or disease variables listed in Tables 1 and 2 as they pertain to naturalistic task success (all ps > .10).
Figure 2.
Bar charts display the interaction between age and both prospective (a) and external (b) compensatory strategy use in predicting naturalistic prospective memory performance in the HIV+ cohort. PMMQ = Prospective Memory for Medications Questionnaire. *p < .05.
DISCUSSION
The present study sought to extend the age-PM paradox to an older HIV-infected population, who represent a growing subpopulation of the HIV epidemic that is at risk for PM impairment (Woods et al., 2010) and declines in everyday functioning (e.g., Thames et al., 2011). Our results showed an interaction between age group and PM task in the HIV+ sample, such that older adults with HIV performed worse than their younger seropositive counterparts on laboratory tasks of PM but performed comparably on a naturalistic PM task. In other words, older HIV+ adults had more difficulty than younger HIV+ adults remembering to execute intentions in the future while constrained in a laboratory setting but were better able to remember to perform an experimenter-decreed task while operating in their daily lives. This study extends the very limited clinical literature on the age-PM paradox (see also Raskin et al., 2011; Will et al., 2009) to individuals living with HIV infection.
Regarding the laboratory PM results, our findings add to a growing body of work suggesting that HIV-associated cognitive deficits may be more pronounced in older adults and, more specifically, a nascent literature examining PM deficits in older HIV-infected adults. In fact, although previous research has demonstrated additive event-based PM deficits related to serostatus and normal aging (Woods et al., 2010), this is the first study to our knowledge to report age-related deficits on time-based PM in HIV. Importantly, these effects remained statistically significant after accounting for the effects of disease characteristics (e.g., AIDS status), suggesting that age-associated PM deficits in HIV are likely not solely due to advanced disease severity and longer duration of infection in older individuals. Further examination of the cognitive mechanisms of time-based PM deficits in older HIV-infected adults is clearly warranted, considering the importance of such deficits across the lifespan as predictors of everyday functioning outcomes (e.g., medication adherence; Woods et al., 2009). For example, future studies that directly assess component processes of time-based PM (e.g., time monitoring, estimation, and production) would be needed to examine these mechanisms further.
In spite of these laboratory-based PM deficits, we found evidence of the age-PM paradox in this sample in that the younger and older HIV+ adults performed comparably on a naturalistic PM task (i.e., a 24-hour telephone task). This suggests that, despite the additional neural injury often associated with aging and HIV infection (e.g., Ernst & Chang, 2004) and associated risk of cognitive impairment (e.g., Cherner et al., 2004), older HIV-infected adults were no different from their younger seropositive counterparts in terms of the ability to remember to execute a future intention outside of the laboratory. These data speak to the robustness of age-related benefits that appear to persevere despite the additional risk for HIV-associated cognitive deficits (e.g., Cherner et al., 2004; Woods et al., 2010). This is commensurate with the literature as well as clinical lore, which has suggested that, in comparison to younger adults with HIV, older HIV+ adults are often more reliable with respect to remembering to take their medication (e.g., Hinkin et al., 2004) and to attend doctor’s appointments than younger adults. Possible mechanisms of naturalistic PM success despite laboratory deficits in HIV have focused on the contributions of environmental structure and compensatory mechanisms. For instance, HIV-infected substance users with a highly structured daily routine tend to be more adherent to their antiretroviral (ARV) regimens (Wagner & Ryan, 2004).
In a series of secondary analyses, the use of PM-based and external mnemonic strategies emerged as a potentially relevant factor in the expression of the age-PM paradox in HIV-infected individuals. In this sample, older HIV-infected adults who successfully performed the naturalistic task reported using PM-based strategies and external aids to help them to remember to take their medications in daily life. This association was not found among younger HIV-infected individuals, suggesting that older adults may need additional support in order to remember PM tasks in their everyday lives, whereas younger adults may rely more so on their raw PM abilities without the use of mnemonic aids. Interestingly, RM-based and internal strategies did not predict naturalistic success in either HIV+ age group, suggesting that there may be something unique to PM-based (e.g., setting an alarm to signal the appropriate time to act) and external (e.g., writing oneself a note) strategies that facilitate the ability to execute future intentions in daily life in this cohort (Vedhara et al., 2004). Although experimental manipulations are needed in order to more fully assess whether strategy use is indeed the mechanism by which individuals are better able to remember to perform tasks in the future, this finding may inform rehabilitation efforts, since it suggests that there may be simple and easily implemented cognitive remediation strategies for PM deficits in HIV infection. The inclusion of mnemonic aids in cognitive rehabilitation efforts for older adults with HIV infection may help to prevent everyday functioning declines, particularly for aspects of daily living that heavily rely upon intact PM abilities (e.g., medication adherence). However, considering the design of the present study, the general use of mnemonic aids may actually be indicative of another factor that was not assessed. For instance, it may be that older adults implement memory strategies into their routines once they notice that long-practiced everyday tasks are becoming more difficult to remember (i.e., increased insight), but younger adults may be using these strategies as a result of a physician recommendation with variable effectiveness due to external factors (i.e., diminished effectiveness of mnemonic aids due to lack of daily routine).
The present study marks the first venture into determining the validity of the age-PM paradox in an HIV-infected sample and used a within-subjects design. The results suggest that the age-PM paradox largely operates comparably in the HIV+ sample as it does in the seronegative group, as indicated by the absence of a significant three-way interaction between age group, serostatus, and PM task type. However, it is important to note that, although not statistically significant, a closer review of the effect sizes shows that the age group difference (i.e., Cohen’s d) on the time-based task in the seronegative sample was more than twice that of the HIV+ group. One possible explanation for the modest attenuation of age-related laboratory task deficits in the seropositive group may be increased noise due to the influence of disease severity and comorbidities in the HIV samples, which may alter associations between demographic factors and cognition. However, the significant age-PM analyses persist even while statistically considering disease characteristics on which the age groups differed, and this concern would hypothetically produce the opposite result (i.e., a larger effect size would be expected in the HIV+ sample due to greater disease severity). A more reasonable explanation may be survivor bias; that is, the older HIV+ group may be composed of healthier individuals who have successfully managed the disease for many years (i.e., so-called “long-term nonprogressors”). Nevertheless, despite the reduced age signal on PM and the relative youth of the older HIV+ individuals, the age-PM paradox is still clearly present in this clinical sample.
Beyond the HIV-infected cohort, this study extends the age-PM paradox to a middle-aged seronegative adult sample, suggesting that although laboratory-based PM deficits may exist earlier than expected in the lifespan, these individuals may still perform daily PM tasks as well as their younger counterparts. In an effort to analyze groups that were demographically comparable, the older seronegative cohort is considered young relative to the aging literature, with an approximate mean age of 56 years compared to typical normal aging cohorts of 65 years and older. Additionally, the younger seronegative group is much older (i.e., mean age of 30 years) than most other studies of the age-PM paradox, where the younger samples are typically drawn from undergraduate populations (e.g., Rendell & Thomson, 1999). Despite performing a standard deviation worse on the laboratory PM task, the HIV− older adults completed the naturalistic task comparably to younger adults. As such, this study replicates the age-PM paradox in a different age range of seronegative adults using a within-subjects design. Moreover, the older HIV+ samples in the present study are considerably younger than samples in other comparable studies examining similar questions in healthy adults (e.g., Rendell & Thomson, 1999). This discrepancy is largely due to the relative youth of the aging HIV+ population, as a result of the timing and nature of the epidemic. However, this would present a conservative bias in terms of performance-based data, whereby we would not find an age effect across the HIV seronegative samples. In fact, our data reflect a stronger than anticipated age effect, which may be in part due to an overzealous attempt to match our older samples on other factors that may impair cognition (e.g., substance dependence). However, in terms of naturalistic task performance, this age discrepancy might represent a Type II error, whereby a significant difference between age groups in the HIV+ sample might be detected in an older sample due to a greater accumulation of risk factors and a relative reduced impact of protective factors.
This study is not without its limitations. For example, this particular HIV-infected sample comprised relatively healthy (e.g., median current CD4 count in 500s), well-educated men, and, therefore, these results may not generalize to all aspects of the HIV epidemic (e.g., women and ethnic minorities). Similarly, the seronegative adult cohort was recruited to be demographically comparable to the seropositive group (e.g., similar rates of psychiatric comorbidities), and as such these results may not generalize to healthier adult samples. As concerns the experimental methods, our measure of naturalistic PM consisted of a single trial with a relatively large time window for a correct response (±15% of the 24-hour target). Although only 3.5% of the sample made “loss of time” errors (i.e., correct responses outside the target time), the liberal scoring criteria used in this study differed from the existing naturalistic PM literature and may have further exacerbated the psychometric differences between our naturalistic and laboratory-based PM tasks. In these ways, the limited demands of the naturalistic trial and possible floor effects on loss of time errors may also increased our risk of Type II error. A greater number of naturalistic trials would have been ideal, as it might have increased the difficulty of the task and perhaps also yielded greater performance variability, thereby increasing its comparability to the MIST time-based score. Extending the issue of comparability to the broader paradox literature, the naturalistic task paradigm used in the present study (e.g., single trial) is not consistent with the multitrial tasks used in some other paradox investigations (e.g., Schnitzspahn et al., 2011), thus limiting our ability to draw direct comparisons across studies. Finally, the laboratory-based and naturalistic trials are not psychometrically comparable on other characteristics of typical PM tasks, such as relative time delay (e.g., short, long) and modality of response (e.g., verbal, action), and therefore may not be equivalent in terms of task reliability and complexity.
In terms of our exploratory analyses, information regarding strategy use was collected in terms of the participants’ everyday habits for medication management and not specifically as it related to this particular PM naturalistic task. Future investigations would benefit from either querying specific strategy use post test or using random assignment to prospectively examine the impact of strategy use on this task. Other factors (e.g., insight, routinization of daily activities) that may feed into the use the mnemonic aids should also be concurrently assessed in future studies, particularly those that may be specific to older individuals with HIV (e.g., increased apathy related to HIV-associated frontal systems deficits; Castellon, Hinkin, & Myers, 2000). More broadly, we elected to explore only one hypothesis (i.e., the use of mnemonic strategies) that may underlie older adults’ success on everyday PM tasks. Considering the complexity of HIV-infected individuals’ medication regimens, the use of external aids (e.g., medication organizers) are often suggested by healthcare providers to their patients and may therefore be of particular relevance to this population to overcome HIV-associated PM deficits. However, other hypotheses regarding the paradox in healthy older adults (e.g., structured lifestyles) may also be applicable and should be tested in future studies.
Acknowledgments
This research was supported by National Institute of Mental Health Grants R01–MH073419 to Steven Paul Woods and P30–MH62512 to Igor Grant. The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Navy, Department of Defense, or the United States Government. Aspects of these data were presented at the 3rd International Conference on Prospective Memory in Vancouver, British Columbia, Canada. The authors thank Catherine L. Carey, Matthew Dawson, Lisa Moran, Ofilio Vigil, Sarah Gibson, and Patricia Riggs for their help with study management.
Footnotes
The San Diego HIV Neurobehavioral Research Center (HNRC) group is affiliated with the University of California, San Diego, the Naval Hospital, San Diego, and the Veterans Affairs San Diego Healthcare System and includes: Director: Igor Grant; Co-Directors: J. Hampton Atkinson, Ronald J. Ellis, and J. Allen McCutchan; Center Manager: Thomas D. Marcotte; Jennifer Marquie-Beck; Melanie Sherman; Neuromedical Component: Ronald J. Ellis (P.I.), J. Allen McCutchan, Scott Letendre, Edmund Capparelli, Rachel Schrier, Terry Alexander, Debra Rosario, Shannon LeBlanc; Neurobehavioral Component: Robert K. Heaton (P.I.), Steven Paul Woods, Mariana Cherner, David J. Moore, Matthew Dawson; Neuroimaging Component: Terry Jernigan (P.I.), Christine Fennema-Notestine, Sarah L. Archibald, John Hesselink, Jacopo Annese, Michael J. Taylor; Neurobiology Component: Eliezer Masliah (P.I.), Cristian Achim, Ian Everall (Consultant); Neurovirology Component: Douglas Richman (P.I.), David M. Smith; International Component: J. Allen McCutchan (P.I.); Developmental Component: Cristian Achim (P.I.); Stuart Lipton; Participant Accrual and Retention Unit: J. Hampton Atkinson (P.I.), Rodney von Jaeger; Data Management Unit: Anthony C. Gamst (P.I.), Clint Cushman (Data Systems Manager); Statistics Unit: Ian Abramson (P.I.), Florin Vaida, Reena Deutsch, Anya Umlauf, Tanya Wolfson.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: Author; 1994. [Google Scholar]
- Bailey PE, Henry JD, Rendell PG, Phillips LH, Kliegel M. Dismantling the “age-prospective memory paradox”: The classic laboratory paradigm simulated in a naturalistic setting. Quarterly Journal of Experimental Psychology. 2010;63:646–652. doi: 10.1080/17470210903521797. [DOI] [PubMed] [Google Scholar]
- Barclay TR, Hinkin CH, Castellon SA, Mason KI, Reinhard MJ, Marion SD, et al. Age-associated predictors of medication adherence in HIV-positive adults: Health beliefs, self-efficacy, and neurocognitive status. Health Psychology. 2007;26:40–49. doi: 10.1037/0278-6133.26.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey CL, Woods SP, Rippeth JD, Heaton RK, Grant I The HNRC Group. Prospective memory in HIV-1 infection. Journal of Clinical and Experimental Neuropsychology. 2006;28:536–548. doi: 10.1080/13803390590949494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellon SA, Hinkin CH, Myers HF. Neuropsychiatric disturbance is associated with executive dysfunction in HIV-1 infection. Journal of the International Neuropsychological Society. 2000;6:336–347. doi: 10.1017/s1355617700633088. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. HIV/AIDS Surveillance Report. Atlanta, GA: US Department of Health and Human Services, Center for Disease Control and Prevention; 2007. [Google Scholar]
- Cherner M, Ellis RJ, Lazzaretto D, Young C, Mindt MR, Atkinson JH, et al. Effects of HIV-1 infection and aging on neurobehavioral functioning: Preliminary findings. AIDS. 2004;18:S27–S34. [PubMed] [Google Scholar]
- Contardo C, Black AC, Beauvais J, Dieckhaus K, Rosen MI. Relationship of prospective memory to neuropsychological function and antiretroviral adherence. Archives of Clinical Neuropsychology. 2009;24:547–554. doi: 10.1093/arclin/acp046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einstein GO, McDaniel MA. Normal aging and prospective memory. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1990;16:717–726. doi: 10.1037//0278-7393.16.4.717. [DOI] [PubMed] [Google Scholar]
- Ellis RJ, Calero P, Stockin MD. HIV infection and the central nervous system: A primer. Neuropsychological Review. 2009;19:144–151. doi: 10.1007/s11065-009-9094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst T, Chang L. Effect of aging on brain metabolism in antiretroviral-naïve HIV patients. AIDS. 2004;18(Suppl 1):S61–S67. [PubMed] [Google Scholar]
- Goetz MB, Boscardin WJ, Wiley D, Alkasspooles S. Decreased recovery of CD4 lymphocytes in older HIV-infected patients beginning highly active antiretroviral therapy. AIDS. 2001;15:1576–1579. doi: 10.1097/00002030-200108170-00017. [DOI] [PubMed] [Google Scholar]
- Gould ON, McDonald-Miszczak L, King B. Metacognition and medication adherence: How do older adults remember? Experimental Aging Research. 1997;23:315–342. doi: 10.1080/03610739708254034. [DOI] [PubMed] [Google Scholar]
- Gupta S, Woods SP, Weber E, Dawson MS, Grant I The HNRC Group. Is prospective memory a dissociable cognitive function in HIV infection? Journal of Clinical and Experimental Neuropsychology. 2010;32:898–908. doi: 10.1080/13803391003596470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry JD, MacLeod MS, Phillips LH, Crawford JR. A meta-analytic review of prospective memory and aging. Psychology and Aging. 2004;19:27–39. doi: 10.1037/0882-7974.19.1.27. [DOI] [PubMed] [Google Scholar]
- Hinkin CH, Hardy DJ, Mason KI, Castellon SA, Durvasula RS, Lam MN, et al. Medication adherence in HIV-infected adults: Effect of patient age, cognitive status, and substance abuse. AIDS. 2004;18:S19–S25. doi: 10.1097/00002030-200418001-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliegel M, Jäger T, Altgassen M, Shum D. Clinical neuropsychology of prospective memory. In: Kliegel M, McDaniel MA, Einstein GO, editors. Prospective memory: Cognitive, neuroscience, developmental, and applied perspectives. New York, NY: Lawrence Erlbaum Associates; 2008. pp. 283–308. [Google Scholar]
- Kliegel M, Martin M, McDaniel MA, Phillips LH. Adult age differences in errand planning: The role of task familiarity and cognitive resources. Experimental Aging Research. 2007;33:145–161. doi: 10.1080/03610730601177395. [DOI] [PubMed] [Google Scholar]
- Martin EM, Nixon H, Pitrak DL, Weddington W, Rains NA, Nunnally G, et al. Characteristics of prospective memory deficits in HIV-seropositive substance-dependent individuals: Preliminary observations. Journal of Clinical and Experimental Neuropsychology. 2007;29:496–504. doi: 10.1080/13803390600800970. [DOI] [PubMed] [Google Scholar]
- Maylor EA. Age and prospective memory. The Quarterly Journal of Experimental Psychology. 1990;42A:471–493. doi: 10.1080/14640749008401233. [DOI] [PubMed] [Google Scholar]
- McDaniel MA, Einstein GO. Strategic and automatic processes in prospective memory retrieval: A multiprocess framework. Applied Cognitive Psychology. 2000;14:S127–S144. [Google Scholar]
- McNair DM, Lorr M, Droppleman LF. Manual for the Profile of Mood States. San Diego, CA: Educational and Industrial Testing Service; 1981. [Google Scholar]
- Moscovitch M. A neuropsychological approach to memory and perception in normal and pathological aging. In: Craik FIM, Trehub S, editors. Aging and cognitive processes. New York, NY: Plenum Press; 1982. pp. 55–78. [Google Scholar]
- Park DC, Kidder D. Prospective memory and medication adherence. In: Brandiamonte M, Einstein G, McDaniel M, editors. Prospective memory: Theories and applications. Hillsdale, NJ: Lawrence Erlbaum Associates; 1996. pp. 369–390. [Google Scholar]
- Patton GW, Meit M. Effect of aging on prospective and incidental memory. Experimental Aging Research. 1993;19:165–176. doi: 10.1080/03610739308253929. [DOI] [PubMed] [Google Scholar]
- Perez JL, Moore RD. Greater effect of highly active antiretroviral therapy on survival in people aged > or = 50 years compared with younger people in an urban observational cohort. Clinical Infectious Diseases. 2003;36:212–218. doi: 10.1086/345669. [DOI] [PubMed] [Google Scholar]
- Psychological Corporation. Wechsler Test of Adult Reading. San Antonio, TX: Author; 2001. [Google Scholar]
- Raskin S, Buckheit C, Sherrod C. Memory for Intentions Screening Test: Manual. Lutz, FL: Psychological Assessment Resources; 2010. [Google Scholar]
- Raskin SA, Woods SP, Poquette AJ, McTaggart AB, Sethna J, Williams RC, Tröster AI. A differential deficit in time-versus event-based prospective memory in Parkinson’s disease. Neuropsychology. 2011;25:201–209. doi: 10.1037/a0020999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendell PG, Craik FIM. Virtual week and actual week: Age-related differences in prospective memory. Applied Cognitive Psychology. 2000;14:S43–S62. [Google Scholar]
- Rendell PG, Thomson DM. The effect of ageing on remembering to remember: An investigation of simulated medication regimens. Australian Journal of Ageing. 1993;12:11–18. [Google Scholar]
- Rendell PG, Thomson DM. Aging and prospective memory: Differences between naturalistic and laboratory tasks. Journal of Gerontology: Psychological Sciences. 1999;54B:256–269. doi: 10.1093/geronb/54b.4.p256. [DOI] [PubMed] [Google Scholar]
- Sacktor N, Skolasky R, Selnes OA, Watters M, Poff P, Shiramizu B, et al. Neuropsychological test profile differences between young and old human immunodeficiency virus-positive individuals. Journal of Neurovirology. 2007;3:203–209. doi: 10.1080/13550280701258423. [DOI] [PubMed] [Google Scholar]
- Schnitzspahn KM, Ihle A, Henry JD, Rendell PG, Kliegel M. The age-prospective memory-paradox: An exploration of possible mechanisms. International Psychogeriatrics. 2011;23:583–592. doi: 10.1017/S1041610210001651. [DOI] [PubMed] [Google Scholar]
- Smith G, Della Sala S, Logie RH, Maylor EA. Prospective and retrospective memory in normal aging and dementia: A questionnaire study. Memory. 2000;8:311–321. doi: 10.1080/09658210050117735. [DOI] [PubMed] [Google Scholar]
- Stoff DM. Mental health research in HIV/AIDS and aging: Problems and prospects. AIDS. 2004;18(Suppl 1):S3–S10. [PubMed] [Google Scholar]
- Thames AD, Kim MS, Becker BW, Foley JM, Hines LJ, Singer EJ, et al. Medication and finance management among HIV-infected adults: The impact of age and cognition. Journal of Clinical and Experimental Neuropsychology. 2011;33:200–209. doi: 10.1080/13803395.2010.499357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valcour V, Paul R, Neuhaus J, Shikuma C. The effects of age and HIV on neuropsychological performance. Journal of the International Neuropsychological Society. 2011;17:190–195. doi: 10.1017/S1355617710001438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valcour V, Shikuma C, Shiramizu B, Watters M, Poff P, Selnes O, et al. Higher frequency of dementia in older HIV-1 individuals: The Hawaii aging with HIV-1 cohort. Neurology. 2004;63:822–827. doi: 10.1212/01.wnl.0000134665.58343.8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedhara K, Wadsworth E, Norman P, Searle A, Mitchell J, MacRae N, et al. Habitual prospective memory in elderly patients with type 2 diabetes: Implications for medication adherence. Psychology, Health, and Medicine. 2004;9:17–27. [Google Scholar]
- Wagner GJ, Ryan GW. Relationship between routinization of daily behaviors and medication adherence in HIV-positive drug users. AIDS Patient Care and STDs. 2004;18:385–393. doi: 10.1089/1087291041518238. [DOI] [PubMed] [Google Scholar]
- Will CM, Rendell PG, Ozgis S, Pierson JM, Ong B, Henry JD. Cognitively impaired older adults exhibit comparable difficulties on naturalistic and laboratory prospective memory tasks. Applied Cognitive Psychology. 2009;23:804–812. [Google Scholar]
- Woods SP, Carey CL, Moran LM, Dawson MS, Letendre SL, Grant I, et al. Frequency and predictors of self-reported prospective memory complaints in individuals infected with HIV. Archives of Clinical Neuropsychology. 2007;22:187–195. doi: 10.1016/j.acn.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Dawson MS, Weber E, Gibson S, Grant I, Atkinson JH, et al. Timing is everything: Antiretroviral nonadherence is associated with impairment in time-based prospective memory. Journal of the International Neuropsychological Society. 2009;15:42–52. doi: 10.1017/S1355617708090012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Dawson MS, Weber E, Grant I The HNRC Group. The semantic relatedness of cue–intention pairings influences event-based prospective memory failures in older adults with HIV infection. Journal of Clinical and Experimental Psychology. 2010;32:398–407. doi: 10.1080/13803390903130737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Iudicello JE, Moran LM, Carey CL, Dawson MS, Grant I. HIV-associated prospective memory impairment increases risk of dependence in everyday functioning. Neuropsychology. 2008;22:110–117. doi: 10.1037/0894-4105.22.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Moran LM, Dawson MS, Carey CL, Grant I. Psychometric characteristics of the Memory for Intentions Screening Test. The Clinical Neuropsychologist. 2008;23:257–270. doi: 10.1080/13854040701595999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Weber E, Weisz BM, Twamley EW, Grant I The HNRC Group. Prospective memory deficits are associated with unemployment in persons living with HIV infection. Rehabilitation Psychology. 2011;56:77–84. doi: 10.1037/a0022753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Composite International Diagnostic Interview (CIDI, Version 2.1) Geneva, Switzerland: World Health Organization; 1998. [Google Scholar]
- Zogg JB, Woods SP, Sauceda JA, Wiebe JS, Simoni JM. The role of prospective memory in medication adherence: A review of an emerging literature. Journal of Behavioral Medicine. 2011 doi: 10.1007/s10865-011-9341-9. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zogg JB, Woods SP, Weber E, Doyle K, Grant I The HNRC Group. Are time- and event-based prospective memory comparably affected in HIV infection? Archives of Clinical Neuropsychology. 2011;26:250–259. doi: 10.1093/arclin/acr020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zogg JB, Woods SP, Weber E, Iudicello J, Dawson MS, Grant I, et al. HIV-associated prospective memory impairment in the laboratory predicts failures on a semi-naturalistic measure of health care compliance. The Clinical Neuropsychologist. 2010;24:945–962. doi: 10.1080/13854046.2010.501343. [DOI] [PMC free article] [PubMed] [Google Scholar]