Fig. 2.
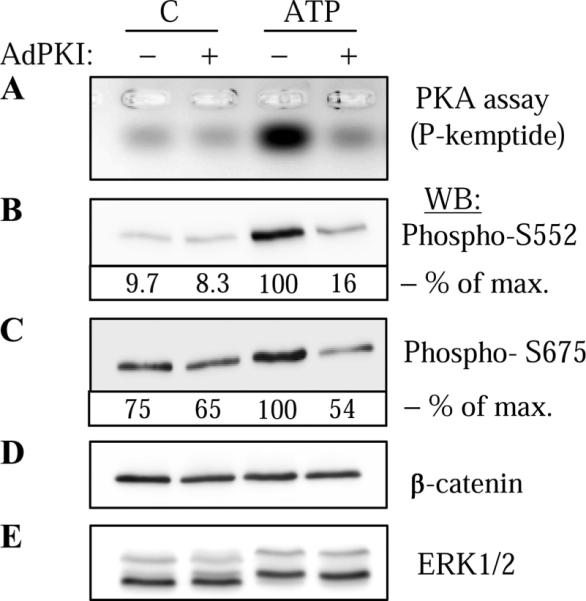
ATP-induced phosphorylation of endogenous β-catenin by PKA in vascular smooth muscle cells (VSMC). VSMC were transduced with control adenovirus (−) or adenovirus encoding PKA inhibitor PKI (Ad-PKI), stimulated with 30 μM ATP for 5 min and lysed. Cell lysates were analyzed for PKA activity (A) or were subjected to Western blotting with phospho-S552 (B) or phospho-S675 (C) β-catenin antibodies as indicated. The equal amounts of endogenous β-catenin were confirmed by Western blotting with β-catenin antibodies (D). For control purposes, ERK1/2 phosphorylation was assessed by electrophoretic mobility shift assay following Western blotting with ERK1/2 antibodies (E). The densitometry of selected blots is shown as % of maximal (max) response to ATP.
