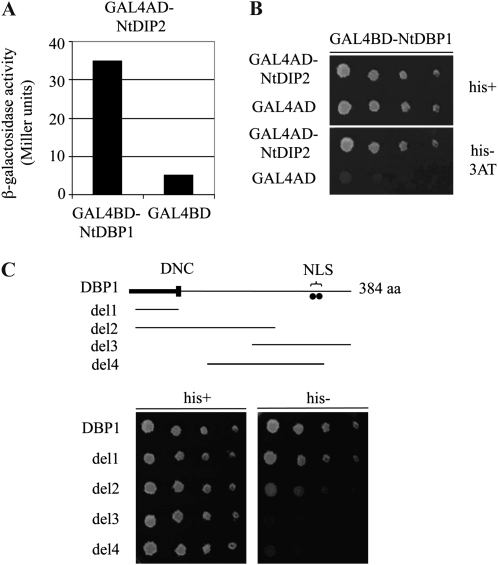
Figure 1.
Interaction of NtDBP1 and NtDIP2 in the yeast two-hybrid system. A, Activation of lacZ reporter gene expression. β-Galactosidase activity was measured from yeast liquid cultures coexpressing NtDBP1 (bait) and NtDIP2 (prey) fused to the GAL4 DNA-binding (BD) and activation (AD) domains, respectively. The empty bait vector was used as a control in conjunction with the NtDIP2-GAL4AD fusion. B, Activation of HIS3 reporter gene expression. Serial dilutions of yeast liquid cultures expressing the referred prey constructs along with the bait GAL4BD-NtDBP1 fusion were plated on medium containing His (his+) and medium lacking His (his−) supplemented with 5 mm 3-amino-triazole (3AT), a competitive inhibitor of the HIS3 gene product, used to eliminate leaky expression of the reporter gene. C, Identification of the domain in NtDBP1 responsible for the interaction with AtDIP2. Different bait constructs comprising different deletions of the NtDBP1 protein were analyzed in the yeast two-hybrid system for the activation of HIS3 reporter gene expression when coexpressed with the GAL4AD-NtDIP2 fusion protein. Yeast growth in the absence of His was observed only in the presence of the N-terminal region of NtDBP1 in the bait construct. aa, Amino acids; DNC, DBP N-terminal core, a characteristic motif of DBP factors involved in DNA binding; NLS, putative bipartite nuclear localization signal.