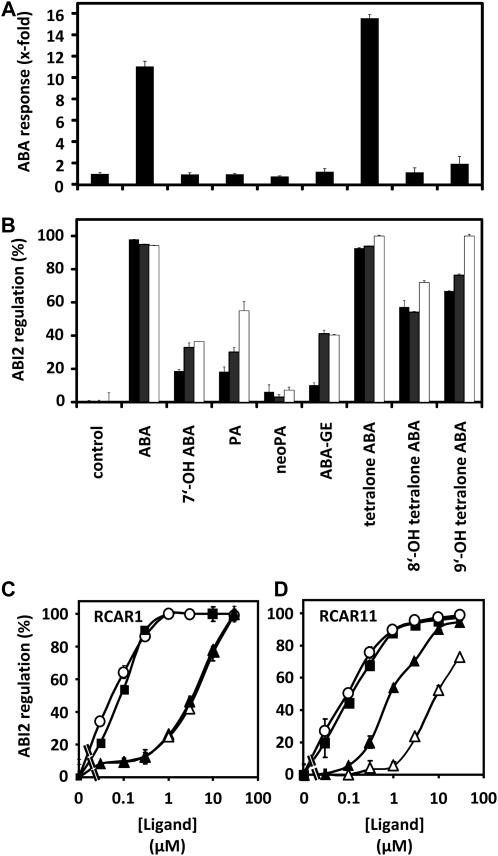
Figure 6.
A, Regulation of the ABA reporter construct pRD29B::LUC by natural ABA catabolites and hydroxy tetralone ABA analogs at 1 μm concentration. Each data point is the mean of three independent transfections. The control represents the protoplast experiment in the absence of ABA or its catabolites. B, Regulation of protein phosphatase activity by RCAR1 (black bars), RCAR3 (gray bars), and RCAR11 (white bars) in the presence of (S)-ABA, ABA catabolites, and ABA tetralone mimics at a ligand concentration of 10 μm. C, Ligand-dependent inhibition of ABI2 in the presence of RCAR1. Half-maximal inhibition of ABI2 occurred at 90 nm (S)-ABA (black squares), 55 nm (S)-tetralone ABA (white circles), 4 μm 8′-OH tetralone ABA (white triangles), and 3.5 μm 9′-OH tetralone ABA (black triangles). D, Ligand-dependent inhibition of ABI2 in the presence of RCAR11. Half-maximal inhibition occurred at 120 nm (S)-ABA (black squares), 95 nm (S)-tetralone ABA (white circles), 8 μm 8′-OH tetralone ABA (white triangles), and 0.9 μm 9′-OH tetralone ABA (black triangles). The 9′-OH tetralone ABA sample was racemic. All phosphatase assays were performed at a PP2C level of 0.05 μm and at a molar ratio of RCAR and ABI2 of 2:1.