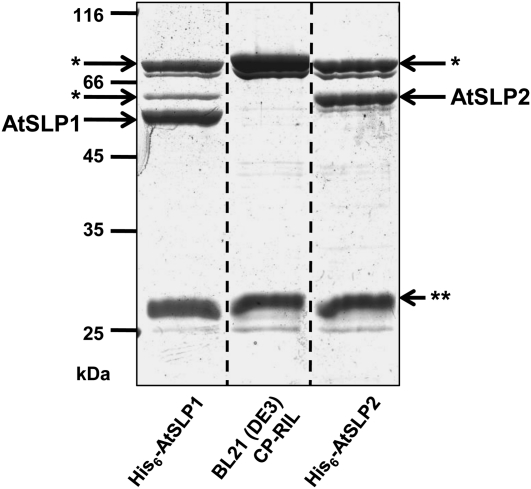
Figure 7.
Twelve percent SDS-PAGE of Ni-NTA-purified His6-AtSLP1, His6-AtSLP2, and uninduced bacterial cell line control eluates stained with colloidal blue. His6-AtSLP1 and His6-AtSLP2 were partially purified by Ni-NTA along with NSCP Ni-NTA-binding proteins isolated in parallel from untransformed, uninduced BL21 (DE3) Codon(+)-RIL E. coli grown under identical conditions. The BL21 (DE3) Codon(+)-RIL E. coli Ni-NTA eluate controlled for nonspecific cleavage of the phosphatase substrate pNPP by NSCP proteins during enzymatic analysis of Ni-NTA-purified His6-AtSLP1 and His6-AtSLP2. The NSCP protein glucosamine-fructose-6-phosphate aminotransferase (asterisks) was identified by mass spectrometry, while double asterisks represent another NSCP protein. Each lane contains 5 μg of concentrated Ni-NTA eluate.