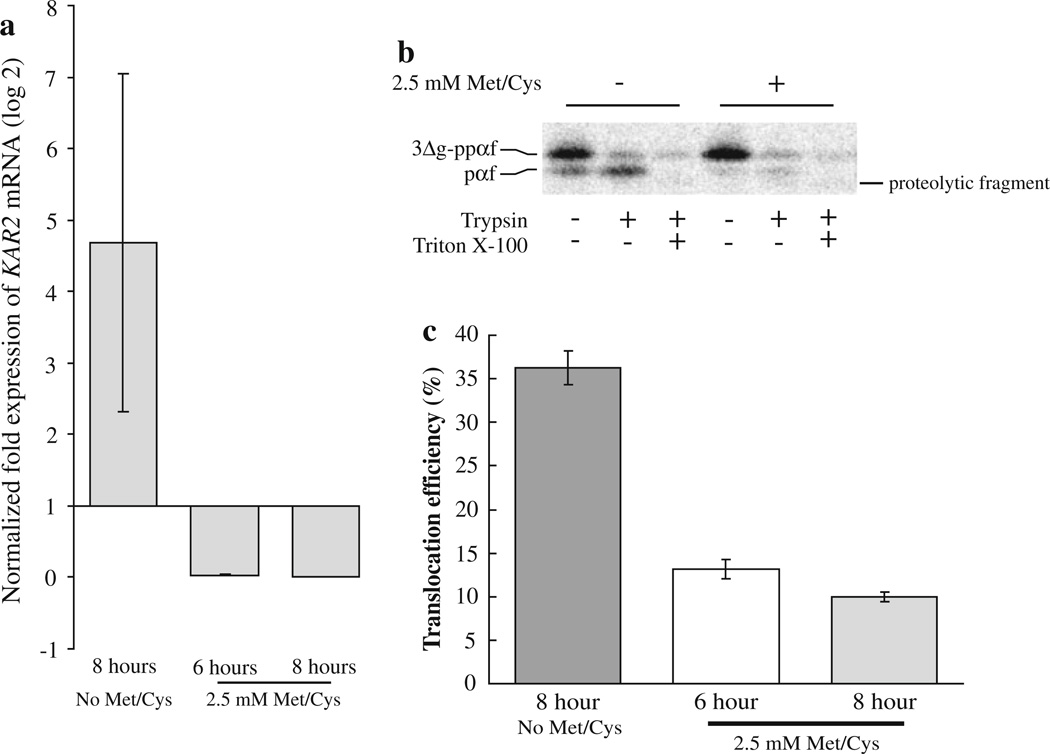
Fig. 6.
Kar2p functions during the translocation of a substrate protein into the C. albicans ER in vitro. BPY116 C. albicans cells were grown in YNB + His (No Met/Cys; inducing conditions) or YNB + His + 2.5 mM methionine and cysteine (repressing conditions) for 6 and 8 h. Total RNA was purified from three ODs of cells, and qRT-PCR was performed to determine the amount of KAR2 mRNA present in the cells (a). The data represent the means of two biological replicates with a total of six technical replicates for the repressed cultures (6 h = 0.028, SD = ±0.010, 8 h = 0.0019, SD = ± 9.9 × 10−4), and the mean of three technical replicates for the induced culture (4.68, SD = ±2.37). KAR2 mRNA levels were normalized to actin and alpha-tubulin genes in each condition, and expression levels are relative to KAR2 expression at the time of inoculation (zero time point, expression = 1). ER microsomes were then purified from strain BPY116 after growth for 8 h in media either containing (+) or lacking (−) 2.5 mM methionine and cysteine. The microsomes were combined with C. albicans cytosol, 35S-labeled S. cerevisiae 3Δg–ppαf and an ATP regenerating system. Translocation assays were performed as described, and typical reactions are shown (b). The presence of trypsin-protected pαf indicates successful translocation. Translocation reactions were also performed using microsomes prepared from strain BPY116 after growth under repressing conditions for 6 and 8 h (n = 15 and 18, respectively). Average translocation efficiencies were compared to translocation efficiencies into microsomes prepared from BPY116 cells grown under inducing conditions for 8 h (n = 18). Standard error of the means are shown as error bars (c)