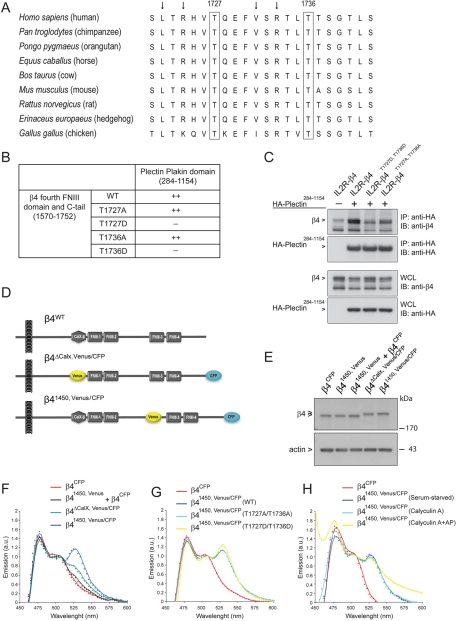
FIGURE 1:
Mimicking phosphorylation of β4 on T1727 or T1736 prevents binding of the C-tail of β4 to the plakin domain of plectin. (A) Amino acid sequence (1721–1742) alignment of β4 from different species using ClustalW. Besides the residues T1727 and T1736, the leucine, valine, and arginines (arrows) are highly conserved. (B) Substitution of T1727 or T1736 on β4 by a phosphomimicking aspartic acid residue resulted in loss of binding of the fourth FNIII domain and C-tail of β4 to the plectin plakin domain in a yeast two-hybrid interaction assay. (C) Coimmunoprecipitation from lysates of COS-7 cells cotransfected with different IL2R/β4 chimeric and HA-tagged plectin plakin-domain constructs showed association of the plakin domain of plectin with the cytoplasmic domains of wild-type β4 and mutant β4 in which T1736 had been replaced by alanine. Replacement of T1736 by aspartic acid eliminated binding. (D) Two β4 Venus-CFP fusion constructs were generated in which a CFP fluorophore was fused to the C-terminus and a Venus fluorophore, which either replaced the nonfunctional CalX domain (β4ΔCalX,Venus/CFP) or was inserted in the CS at position 1450 (β41450,Venus/CFP), where in the β4B variant an additional 53 amino acids are located. (E) COS-7 cells transiently transfected with β4CFP, β41450,Venus, a combination of β4CFP and β41450,Venus, β4ΔCalX, Venus/CFP, or β41450,Venus/CFP were lysed, and the levels of the different β4 Venus-CFP fusion proteins were analyzed by immunoblotting with polyclonal antibodies against β4. (F) COS-7 cells transiently expressing the indicated β4 Venus-CFP fusion proteins were serum starved overnight and lysed. The fluorescence emission spectra of the cell lysates upon excitation at 390 nm were recorded using a spectrophotometer and plotted. The expression of β41450,Venus/CFP resulted in a FRET signal at 527 nm, indicating that the C-tail is in close proximity to the CS of β4. (G, H) Serum-starved COS-7 cells transiently expressing the indicated β4 Venus-CFP fusion proteins were either left untreated or treated with calyculin A (50 nM) in growth medium (DMEM + 10% FCS) for 25 min and lysed. An aliquot of the lysate from the calyculin A–treated cells was incubated with alkaline phosphatases (AP; 60 U/ml) for 30 min at 37°C. The emission spectra of the cell lysates after excitation at 390 nm were analyzed and plotted. Substitution of T1727 and T1736 by alanine or aspartic acid, or treatment of lysates with calyculin A or AP, did not influence the emission spectra significantly.