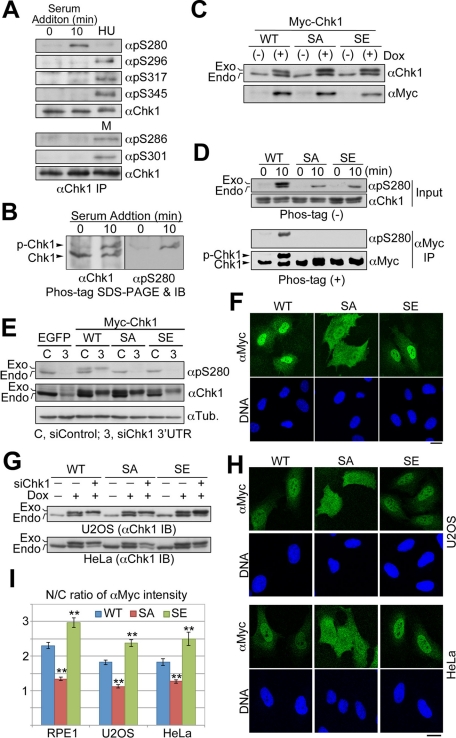
FIGURE 2:
Chk1 is phosphorylated specifically at Ser-280 in response to serum stimulation. (A, B) Endogenous Chk1 was immunoprecipitated from cells stimulated by 10% serum for 0 or 10 min, HU-treated or mitotic (M) cells. Each immunoprecipitate was subjected to the SDS–PAGE with (B) or without (A) Mn2+-Phos-tag, followed by immunoblotting with the indicated antibody. (C) Establishment of each Tet-On RPE1 cell line. Cells were treated with (+) or without (−) 2 ng/ml doxycycline (Dox) for 48 h. SA or SE indicates Myc-tagged Chk1 mutated at Ser-280 to Ala or Glu, respectively. (D) Tet-On RPE1 cell line was cultured in the serum-free medium containing 5 ng/ml Dox for 48 h. After serum starvation, cells were incubated in the growing medium for 0 or 10 min. After treatment, cells were subjected to αMyc immunoprecipitation. The immunoprecipitate (αMyc IP) or a fraction of each cell extract (Input) was subjected to the SDS–PAGE with (+) or without (−) Mn2+-Phos-tag, followed by immunoblotting, respectively. (E–I) Each Tet-On cell line was transfected with control or Chk1 3′ UTR siRNA according to the forward transfection procedures (Invitrogen). At 4 h after transfection, the medium was replaced with the fresh growing medium containing Dox. At 24 h after transfection, cells were analyzed by immunoblotting (E, G) or immunocytochemistry (F, H, I). In E, we used Tet-On RPE1 cell line expressing EGFP as a negative control. In G, each Tet-On cell line was also incubated with or without Dox for 24 h in order to evaluate inducible expression of each Myc-Chk1. The N/C ratio of αMyc intensity is shown. Data represent mean ± SEM for at least 20 cells in each cell group, **p < 0.01 vs. WT-replacing cells (I). Similar results were obtained using another Chk1 3′UTR sequence (unpublished data). Scale bar, 10 μm (F, H).