Abstract
Background
Articular cartilage of young healthy individuals is dynamic and responsive to loading behaviors. The purpose of this study was to evaluate the relationship of cartilage T1ρ and T2 relaxation times with loading kinetics during jumping tasks in healthy young individuals.
Methods
Fourteen healthy subjects underwent: 1) motion analysis while performing a unilateral hopping task and bilateral drop jumping task; and 2) quantitative imaging using a 3 Tesla MRI for T1ρ and T2 relaxation time analysis. Three dimensional net joint moments and angular impulse was calculated using standard inverse dynamics equations. Average T1ρ and T2 relaxation times and medial-lateral ratios for each were calculated. Multiple regression was used to identify predictors of cartilage relaxation times.
Findings
Average knee flexion moment during hopping was observed to best predict overall T1ρ (R2=.185) and T2 (R2=.154) values. Peak knee adduction moment during a drop jump was the best predictor of the T1ρ medial-lateral ratio (R2=.220). The T2 medial-lateral ratio was best predicted by average internal rotation moment during the drop jump (R2=.174).
Interpretation
These data suggest that loads across the knee may affect the biochemistry of the cartilage. In young healthy individuals, higher flexion moments were associated with decreased T1ρ and T2 values, suggesting a potentially beneficial effect. The medial-to-lateral ratio of T1ρ and T2 times appears to be related to the frontal and transverse plane joint mechanics. These data offer promising findings of potentially modifiable parameters associated with cartilage composition.
Keywords: MRI, Motion analysis, Biomechanics, Cartilage, Osteoarthritis
1. Introduction
Osteoarthritis (OA) is a degenerative disease that is characterized by cartilage thinning and compositional changes. It is estimated that 20 million individuals in the United States are living with the disease with an annual cost of over 15 billion dollars (Jackson et al., 2001; Lawrence et al., 1998) and that 20–35% of individuals 60–64 years old will develop knee OA over the next 10 years. (Holt et al., 2010).
Of particular interest to OA research is the external knee adduction moment (KAM) which has been found to be elevated in subjects with the disease.(Astephen et al., 2008; Gok et al., 2002) The KAMis a kinetic variable that describes the frontal plane torque about the knee joint. Approximately half of the variability in KAMis explained by the tibiofemoral mechanical axis (Hurwitz et al., 2002), and the other half is related to dynamic variables of loading. While average and peak KAM have been found to be associated with OA to varying degrees, Thorp and colleagues (Thorp et al., 2006) have suggested that knee adduction angular impulse is amore sensitive kinetic variable at identifying early OA.
Several studies have investigated the relationship of KAM and angular impulse to morphological characteristics of cartilage in healthy and OA subjects. (Creaby et al., 2010; Jackson et al., 2004; Koo and Andriacchi, 2007; Vanwanseele et al., 2010) While Jackson and colleagues (Jackson et al., 2004) reported no relationship between peak KAM and cartilage thickness in healthy females, Koo and Andriacchi (Koo and Andriacchi, 2007) reported a significant positive correlation between peak KAM during gait and the medial-lateral cartilage thickness ratio (M/L ratio) in both the femur and tibia in healthy young males. Two recent studies reported that KAM is not related to cartilage volume, thickness or the medial-lateral thickness ratio in subjects with OA. (Creaby et al., 2010; Vanwanseele et al., 2010). However, one of these studies did observe a significant relationship between the knee adduction angular impulse and the medial-lateral thickness ratio of femoral cartilage.(Vanwanseele et al., 2010).
Additional studies have reported that KAM is related to other markers of OA pathology including medial meniscus tears (Davies-Tuck et al., 2008) and cartilage defects.(Creaby et al., 2010) Kinematic and kinetic patterns, such as KAM, during functional tasks such as gait and jumping are remarkably consistent within individuals and have been found to be very reliable from session to session making them appropriate for these types of investigations (ICC’s: 0.872–0.959 in the sagittal plane, and 0.675–0.860 in the frontal plane). (Birmingham et al., 2007; Milner et al., 2011) However, one possible limitation from each of these studies is that all have limited their investigation to frontal plane knee kinetics during gait, when loading events are relatively small. Perhaps stronger relationships would be observed during more dynamic tasks such as running or jumping when the moments across the knee joint are much larger, and include knee flexion moments as well as transverse plane moments. Taken together, biomechanical variables such as KAM and knee adduction angular impulse appear to play a role in cartilage morphology, but further work is required to clarify the relationship.
One possible explanation for this uncertainty, particularly in the healthy cohorts, is that morphological degeneration is a fairly late-stage characteristic of OA. Recent MRI advancements have the potential to identify early biochemical changes in the cartilage composition such as subtle changes in proteoglycan content, collagen organization and tissue hydration prior to thickness changes, and include T1ρ, T2 relaxation time mapping, and delayed gadolinium enhanced MRI of cartilage (dGEMRIC).(Bashir et al., 1997; Regatte et al., 2006; Stahl et al., 2009) T1ρ relaxation time mapping, or spin–lattice relaxation in the rotating frame, probes the interaction of protons with the macromolecular environment, primarily proteoglycans. Loss of proteoglycan within the cartilage matrix has been reported as the first changes in cartilage degeneration (Aigner et al., 2006), which results in increased T1ρ relaxation times. T2 relaxation time mapping, or spin-spin relaxation is associated with tissue hydration and collagen anisotropy. In early OA, the tissue increases in water content and the collagen becomes disorganized, both of which result in an elevated T2 relaxation time. Acute loading of cartilage has a direct influence on both T1ρ and T2 relaxation times in healthy and OA subjects (Souza et al., 2010a), and experimental varus loading has been shown to significantly reduce medial compartment T2 times in porcine knees. (Shiomi et al., 2010) However, very few studies have investigated the influence of loading behaviors on these imaging parameters, and no studies to date have evaluated the relationship of knee kinetics during dynamic tasks with advanced quantitative MRI relaxation time mapping of cartilage.
Therefore the purpose of this study is to evaluate the relationship of cartilage T1ρ and T2 relaxation times of the knee with loading kinetics in healthy young individuals during hopping and jumping tasks. We hypothesize that knee kinetics will be predictive of cartilage relaxation times, indicating that the biochemical structure of the knee articular cartilage potentially reflects the magnitude of loads generated across the knee joint during dynamic tasks.
2. Methods
2.1. Subjects
A total of 28 knees from 14 young healthy subjects were included in the current study (Table 1). All subjects were recreationally active. Subjects were recruited from the student population at the University of California, San Francisco.
Table 1.
Subject characteristics.
| Demographics | |
|---|---|
| Gender | 7 males, 7 females |
| Age mean (SD) | 22.7 (3.3) years |
| BMI mean (SD) | 22.1 (2.3) kg/m2 |
Exclusion criteria included: 1) current hip, knee, or ankle symptoms (pain, swelling, etc.); 2) history of knee surgery; 3) history of injury to knee ligaments or menisci; 4) change in physical activity levels over the preceding 3 months prior to data collection; 5) any neurologic or vestibular disorders that would influence the subjects ability to perform hopping or jumping tasks; and 6) implanted biological devices that could interact with the magnetic field (i.e. pacemakers, cochlear implants, or ferromagnetic cerebral aneurysm clips).
2.2. Procedures
All subjects participated in two separate data collection sessions (Fig. 1). First, subjects underwent functional movement testing including a fixed-frequency hopping task and a fixed-height drop jump task. Next, on a separate day, MR imaging to assess knee cartilage relaxation times was performed. Data collection sessions were separated to avoid the acute effects of activity on relaxation time mapping which has been reported in the literature.(Luke et al., 2010) Furthermore, subjects were instructed to perform “usual” physical activity on the day prior to MRI acquisition and to refrain from any physical activity (aside from walking) during the day of MRI acquisition, and all scans were performed in the morning on the day of data collection to control for the diurnal effects of loading on cartilage relaxation times. Motion analysis testing was performed at the Human Performance Center at UCSF’s Orthopaedic Institute, and imaging was performed at the Department of Radiology and Biomedical Imaging MRI facilities at the University of California, San Francisco. Prior to testing, all procedures were explained and each subject signed a human subject’s consent form as approved by the Committee on Human Research at the University of California, San Francisco. After agreeing to participate, subject age, height and weight were recorded.
Fig. 1.
Photo of a subject performing a hopping task (a), the reconstructed skeleton from motion analysis data (b), and photo of MRI acquisition set up (c).
2.2.1. Kinematic evaluation
Three-dimensional motion analysis was performed using a computer-aided video motion analysis system (Vicon, Oxford Metrics LTD. Oxford, England). Retro-reflective marker coordinate data were sampled at 250 Hz and used to quantify lower extremity kinematics. Ground reaction forces were obtained using two AMTI force plates (Model #OR6-6-1, Newton, MA) at a rate of 1000 Hz. Net joint moment were calculated using standard inverse dynamics equations in Visual 3D software(C-Motion, Rockville, MD).
Calibration markers were placed bilaterally on the greater trochanters, medial and lateral femoral epicondyles, medial and lateral malleoli, and 1st and 5th metatarsal heads, in order to locate the segment origins. In addition, clusters of rigid reflective tracking markers were securely placed bilaterally on the lateral surface of the subject’s thighs, legs and heel shoe counters. Additional tracking markers were placed on each anterior superior iliac spine, iliac crest, and the L5/S1 inter-spinous space. Once the markers were placed, a standing trial was collected. After a standing trial, the calibration markers were removed. The tracking markers remained on the subject for the remainder of the motion analysis collection session.
All subjects performed two tasks: 1) a 1.67 Hz hopping task, and 2) a 30.5 cm drop jump task. First, for the hopping task, subjects were positioned on the force platform and instructed to continuously hop on their left lower extremity. They were instructed to “clear your foot” on each successive hop and remain within the boundaries of the force platform (51 cm long×46 cm wide). Tempo was regulated via a digital metronome. Once the subject reached a steady pace of hopping that matched the metronome beats, data were collected for 20 successive hops. A successful hop was considered one in which the foot of the subject landed completely within the boundary of the force platform, followed by the foot completely leaving the force platform(as measured by the ground reaction forces). Three trials of 20 hops were acquired on each leg.
Next, subjects performed a drop jump task as described by Pollard and colleagues.(Pollard et al., 2006) Each subject started from a standing position on a 30.5 cm platform and was instructed to drop onto 2 force plates (1 for each foot) with both feet simultaneously and upon landing, immediately jump upward as high as possible. Four trials of data were collected for each subject.
2.2.2. Magnetic resonance imaging
Imaging was performed on both knees of each subject using a 3 T GE MR scanner (Signa HDx, General Electric, Milwaukee, WI) and an 8-channel phased-array knee coil. The following image sequences were acquired: 1) morphological images: 3D sagittal high-resolution fat-saturated spoiled-gradient echo images (SPGR): TR: 20.1 ms, TE: 5.9 ms, FOV: 140mm, matrix: 512×512, slice thickness: 2 mm; 2) sagittal T1ρ relaxation time mapping images based on spin-lock magnetization preparation followed by 3D SPGR acquisition(Li et al., 2004): time of spin-lock: 0, 10, 40, 80 ms, spin-lock frequency: 500 Hz, TR: 9.1 ms, TE: 3.3 ms, magnetization recovery time: 1500ms, FOV: 140mm, matrix: 256×192, slice thickness: 4 mm; and 3) sagittal T2 relaxation time mapping images based on MLEV magnetization preparation followed by 3D SPGR acquisition(Li et al., 2009): parameters were identical to T1ρ parameters with the exception of preparation TEs: 2.8, 13.2, 23.7, 44.5 ms.
Subjects were positioned in the MR scanner in a supine position. The left knee was positioned in a phased-array knee coil and supported with cushions to prevent movement. High-resolution SPGR, T1ρ and T2 mapping sequences were acquired. All procedures were repeated on the subjects’ right knee. Total imaging time for bilateral knees was approximately 50 minutes.
2.3. Data analysis
Reflective markers were labeled manually within the VICON Nexus software and then imported into Visual 3D software to quantify three-dimensional kinetics using standard inverse dynamics equations. The kinetic variables of interest were peak and average external net joint moments and angular impulse at the knee in the sagittal, frontal and transverse planes during the stance phase of both the hopping task and the drop jump task. The stance phase was identified by the presence of a ground reaction force from the force plate data.
Relaxation time maps were calculated on a voxel-by-voxel basis using established fitting routines.(Li et al., 2007) Cartilage segmentations were performed on downsampled registered high-resolution SPGR images and overlaid on the relaxation time maps for quantification. Five cartilage plates were segmented: medial and lateral femoral condyles, medial and lateral tibiae, and patella. The data was reduced by evaluating two separate dependent variables for each relaxation time map: 1) Pixel-weighted average T1ρ or T2 time across all compartments expressed in ms, and 2) T1ρ or T2 medial-lateral (M/L) unitless ratio which was calculated as: (average medial femoral cartilage+ average medial tibia cartilage) / (average lateral femoral cartilage+ average lateral tibia cartilage).
2.4. Statistical analysis
The relationships of kinetic variables and MRI relaxation time variables were explored using a linear univariate regression. Any kinetic variables that revealed a significant relationship to cartilage imaging variables, as well as gender, age, and BMI were entered into a step-wise multiple regression to determine the best predictors of cartilage relaxation times. All statistics were performed in SPSS with p<0.05.
3. Results
Average and standard deviations for all kinetic variables for both tasks are presented in Table 2. Overall T1ρ or T2 relaxation times for each knee included in the study are presented in Fig. 2b & c. The average time between MRI and motion analysis acquisition was 15 days with a range between 1 and 66 days.
Table 2.
Kinetic Variables.
| Kinetic Variable | Average | Standard Deviation |
|---|---|---|
| Hopping | ||
| Average Flexion Moment (Nm/kg) | 1.29 | 0.22 |
| Peak Flexion Moment (Nm/kg) | 2.78 | 0.63 |
| Average Ab/Adduction Moment (Nm/kg) | 0.39 | 0.32 |
| Peak Adduction Moment (Nm/kg) | 0.85 | 0.53 |
| Average Internal /External Rotation Moment (Nm/kg) | 0.32 | 0.13 |
| Peak Internal Rotation Moment (Nm/kg) | 0.71 | 0.32 |
| Sagittal Plane Impulse (Nm*s/kg) | 0.52 | 0.09 |
| Frontal Plane Impulse (Nm*s/kg) | 0.13 | 0.11 |
| Transverse Plane Impulse (Nm*s/kg) | 0.12 | 0.04 |
| Drop Jump | ||
| Average Flexion Moment (Nm/kg) | 1.15 | 0.22 |
| Peak Flexion Moment (Nm/kg) | 2.09 | 0.46 |
| Average Ab/Adduction Moment (Nm/kg) | −0.06 | 0.17 |
| Peak Abduction Moment (Nm/kg) | −0.45 | 0.22 |
| Peak Adduction Moment (Nm/kg) | 0.27 | 0.16 |
| Average Internal /External Rotation Moment (Nm/kg) | −0.04 | 0.11 |
| Peak Internal Rotation Moment (Nm/kg) | −0.34 | 0.18 |
| Peak External Rotation Moment (Nm/kg) | 0.22 | 0.16 |
| Sagittal Plane Impulse (Nm*s/kg) | 0.54 | 0.19 |
| Frontal Plane Impulse (Nm*s/kg) | 0.02 | 0.08 |
| Transverse Plane Impulse (Nm*s/kg) | 0.02 | 0.06 |
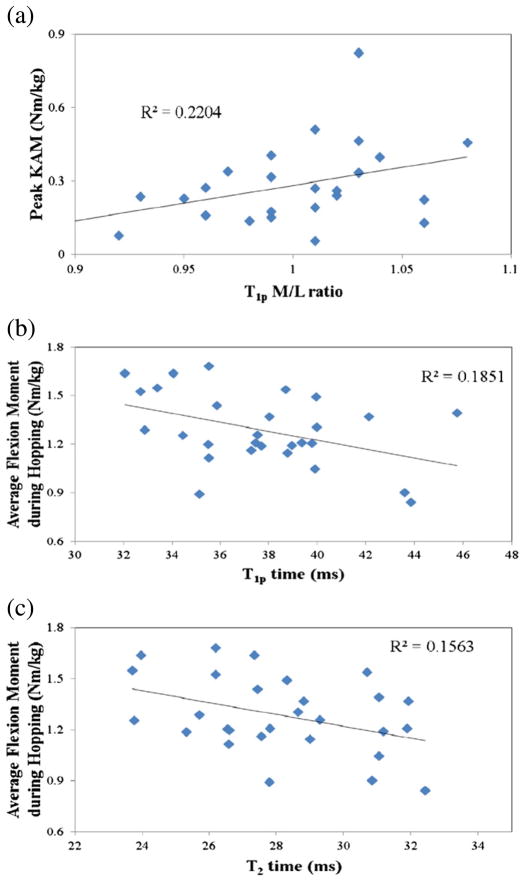
Fig. 2.
Relationship between peak knee adduction moment (KAM) during a drop jump and T1ρ medial-lateral (M/L) ratio (a), and average knee flexion moment during hopping and T1ρ (b) and T2 times (c).
3.1. Overall T1ρ relaxation times
Significant correlations were observed between overall average T1ρ times and 1) average knee flexion moment during hopping and 2) peak knee valgus moment during the drop jump. (Table 3). When these variables were entered into a stepwise regression model along with age, gender, and BMI, two significant models were identified as being significant predictors of overall T1ρ values: 1) average knee flexion moment during hopping and 2) a combination of average knee flexion moment during hopping and peak knee valgus moment during the drop jump (Table 4).
Table 3.
Correlation analysis of MR relaxation times (only significant relationships shown).
| MR variables | Kinetic variables | Univariate Analysis |
|---|---|---|
| Overall T1ρ | Average flexion moment during hopping | −.430 |
| Peak abduction moment during a drop jump | .377 | |
| T1ρM/L ratio | Peak adduction moment during a drop jump | .470 |
| Average frontal moment during a drop jump | .414 | |
| Sagittal plane impulse during drop jump | .449 | |
| Overall T2 | Average flexion moment during hopping | −.395 |
| T2M/L ratio | Average rotational moment during a drop jump | .418 |
Table 4.
Regression analysis of MR relaxation times.
| MR variable | Regression model | R2 value | Beta | P-value |
|---|---|---|---|---|
| Overall T1ρ | Average knee flexion moment during hopping | .185 | .430 | 0.022 |
| Average knee flexion moment during hopping | .321 | .423 | 0.008 | |
| +Peak abduction moment during a drop jump | .368 | |||
| T1ρ M/L | Average knee flexion moment during hopping | .156 | .395 | 0.037 |
| Average knee flexion moment during hopping | .394 | .826 | 0.002 | |
| +Gender | .650 | |||
| Overall T2 | Peak adduction moment during a drop jump | .220 | .470 | .012 |
| T2 M/L | Average rotational moment during a drop jump | .174 | .418 | .027 |
3.2. T1ρ M/L ratio
Significant correlations were observed between the M/L ratio of T1ρ times and the peak knee adduction moment during the drop jump, average frontal plane knee moment during the drop jump, and impulse in the sagittal plane during the drop jump. Stepwise multiple regression identified peak knee adduction moment as the only predictor of the T1ρ M/L ratio (Table 4, Fig. 2).
3.3. Overall T2 relaxation times
A significant correlation was observed between overall T2 values and average knee flexion moments during hopping (Table 3). When this was entered into a stepwise multiple regression along with age, gender and BMI, two models were identified as being significant predictors of overall T2 relaxation times: 1) average flexion moment during hopping, and 2) a combination of average flexion moment during the hopping and gender (Table 4).
3.4. T2 M/L ratio
With regard to the T2 M/L ratio, a significant correlation was identified with average internal rotation moments during the drop jump (Table 3). Regression model identified the average internal rotation moment during the drop jump as the only significant predictor of the T2 M/L ratio (Table 4).
4. Discussion
This study explored relationships between MR variables of knee cartilage biochemistry and loading variables during jumping tasks to determine if movement strategies would be predictive of cartilage composition. We observed significant relationships between several loading variables and tissue relaxation times in a cohort of young healthy individuals. These data offer promising findings of potentially modifiable variables associated with cartilage biochemistry.
Greater sagittal plane loading during hopping was associated with lower T1ρ values in our cohort. The single best predictor of overall T1ρ relaxation times was the average knee flexion moment during hopping. The repeated hopping task allows subjects to utilize various movement strategies to accomplish the desired result and requires a combination of hip extension, knee extension, and ankle plantarflexion effort. Previous literature has shown that differences in these movement strategies exist in the young active population and may be related to overuse injuries.(Souza et al., 2010b) We observed that subjects that loaded their knee more, both in the sagittal and frontal plane (valgusmoment), during hopping tended to have lower T1ρ values. As T1ρ relaxation time has been linked to proteoglycan content in articular cartilage (Li et al., 2010; Wheaton et al., 2005), this would suggest that those individuals with higher loading in the sagittal plane have greater amount of proteoglycan, which may be protective against cartilage degeneration.
Consistent with Koo and Andriacchi (Koo and Andriacchi, 2007), we observed a significant relationship between the peak KAM and MRM/L ratio variables. While these authors and other investigators have previously reported correlations of varying strength (R2=0.04 - 0.4) between KAM and the M/L cartilage thickness ratio during walking, (Koo and Andriacchi, 2007; Vanwanseele et al., 2010) we observed a similar relationship (R2=.22) between KAM and T1ρ M/L relaxation time ratio during a jumping task. Interestingly, the previous studies data showed that a larger adduction moment was associated with thicker medial cartilage compared to the lateral side, suggesting that the increased load on the medial compartment from the adduction moment is associated with a hypertrophic or perhaps a swelling response. Similarly, our data shows elevated T1ρ values in the medial compartment compared to the lateral compartment in subjects that generated greater adduction moments – indicative of lower protoeoglycan content and potentially poorer ability to withstand compressive loads. It is important to reiterate that this relationship was observed between cartilage composition and a jumping task — something that, while repeatable and consistent, is performed at a much lower frequency than loading events during walking. Further research should expand this analysis to include more habitual loading patterns such as walking to evaluate if an even stronger relationship is observed. Nonetheless, these data show that loading strategies during dynamic tasks are significantly related to articular cartilage metrics.
Similar to overall T1ρ relaxation times, overall T2 times were found to be related to sagittal plane moments during dynamic tasks. Specifically, an increased knee flexion moment during hopping was found to be associated with lower T2 values. T2 times have been related to collagen content and organization (Xia, 1998) and are observed to be elevated in subjects with OA (Li et al., 2007; Stahl et al., 2007), suggesting again that increased loading in the sagittal plane is associated with denser, more organized cartilage composition in young active adults. A second model was identified for overall T2 times which included gender as an additional predictor along with knee flexion moment during hopping. These are interesting findings as a gender predisposition for OA has been previously identified (Jiang et al., 2011) and warrants further investigation. However, the current study with its limited sample size is unable to make appropriate gender comparisons.
With regard to the T2 M/L ratio, it was observed that the average transverse plane moment during the drop jump task was the only significant predictor of cartilage relaxation times. This may suggest that collagen organization is related to rotational or shear forces during functional tasks.
Taken together, the results of the current study suggest that loading strategies during hopping and jumping tasks, even those performed occasionally are significantly associated with articular cartilage composition of the knee. However, it should be stressed that on average, only ~25% of the variability in MR relaxation times of cartilage were explained by knee kinetic variables during jumping tasks. This implies that 75% of the variability is related to other variables such as structural characteristics and very likely genetic make-up. Nonetheless, these data offer a promising source of potential intervention as loading behaviors have been reported to be modifiable through various strategies. (Noehren et al., 2010; Snyder et al., 2009) If these data, along with findings from habitual tasks such as walking, can identify movement patterns as being either injurious or protective of cartilage degeneration, clinicians can use this information to develop retraining and strengthening protocols to optimize cartilage health in young adults and prevent or slow cartilage disease in patients with OA.
Knee impulse in any plane was not observed to be a significant predictor in the current study. While reported to be a more sensitive predictor of cartilage degeneration than KAM (Thorp et al., 2006), it does not appear to be a significant predictor of cartilage relaxation timing in young healthy adults. Additionally, with the exception of a relationship between gender and overall T2 values, demographic variables of age, gender and BMI were not identified as predictors of cartilage relaxation times. However, given the homogeneous nature of the sample of young healthy subjects, this is not surprising and is more likely a reflection of the sample selection. Future studies should continue to consider these variables as each have been clearly linked to cartilage disease in previous publications. (Jiang et al., 2011)
Several limitations need to be noted with regard to the current study. First, we investigated two specific jumping tasks, which may or may not be common tasks for our subjects. All of our subjects were noted to be recreationally active, but no information of type of activities performed was recorded. Additionally, while all subjects reported being recreationally active, we did not gather information about habitual volume of activities performed. It is likely that subjects that performed several hours of activity per day during development would have different cartilage composition that subjects that were relatively sedentary. Another potential limitation is that the MRI and motion analysis collection sessions were not performed in immediate succession, with an average of about 2 weeks between sessions. However, as all of these subjects were young and healthy and none sustained injuries during this time, our experiences with longitudinal data on healthy controls suggests that this time lapse would not result in significant change in cartilage relaxation times. Next, we chose to only report overall relaxation times and compartmental ratios. While several significant relationships were observed between specific cartilage plates and loading kinetics, we chose to take a conservative approach in this first investigation in this area as we did not want to increase our type I error for correlation analysis. Clearly, specific cartilage plates that are prone to cartilage disease (medial tibia, patella) should be looked at individually in future investigations. Finally, as noted above, significant limitations are linked to our study sample. Our small cohort had a very narrow age range and these data are undoubtedly not generalizable to older or younger populations. Nonetheless, these data offer preliminary findings that should be verified through larger studies with sufficient power to evaluate additional variables such as age, gender, and BMI to determine the unique contribution of loading kinetics to cartilage relaxation times.
The clinical implications of these data are promising but far too preliminary to substantiate firm conclusions. While we observed that in young healthy subjects, T1ρ and T2 values varied significantly between individuals – 30–35% of the average relaxation time – additional studies are needed to clarify these findings. It may be that individuals that produce large sagittal plane loads during dynamic tasks have a more densely rich cartilage matrix (higher proteoglycan and collagen content) as indicated by the observed lower T1ρ and T2 values. If this were true, it may lead to health and wellness centers offering jumping re-education programs, similar to those offered for ACL injury prevention strategies in young athletes. However, further work, including the evaluation of other more habitual loading tasks such as walking are needed to fully understand the relationship between loading mechanics and cartilage biochemical structure.
In summary, these data suggest that higher loads across the knee are significantly related to composition of the articular cartilage. In young healthy individuals, increased flexion moments during hopping were associated with decreased T1ρ and T2 values, suggesting a beneficial effect in this cohort. Additionally, the medial-to-lateral ratio of T1ρ and T2 times appears to be related to the frontal and transverse plane joint mechanics. These data offer promising findings of potentially modifiable determinants of cartilage biochemistry.
Acknowledgments
This study was supported by grant number P30 AR058899.
Footnotes
Competing Interest Statement
There are no competing interests to declare.
References
- Aigner T, Sachse A, Gebhard PM, Roach HI. Osteoarthritis: pathobiology-targets and ways for therapeutic intervention. Adv Drug Deliv Rev. 2006;58:128–149. doi: 10.1016/j.addr.2006.01.020. [DOI] [PubMed] [Google Scholar]
- Astephen JL, Deluzio KJ, Caldwell GE, Dunbar MJ. Biomechanical changes at the hip, knee, and ankle joints during gait are associated with knee osteoarthritis severity. J Orthop Res. 2008;26:332–341. doi: 10.1002/jor.20496. [DOI] [PubMed] [Google Scholar]
- Bashir A, Gray ML, Boutin RD, Burstein D. Glycosaminoglycan in articular cartilage: in vivo assessment with delayed Gd(DTPA)(2-)-enhanced MR imaging. Radiology. 1997;205:551–558. doi: 10.1148/radiology.205.2.9356644. [DOI] [PubMed] [Google Scholar]
- Birmingham TB, Hunt MA, Jones IC, Jenkyn TR, Giffin JR. Test-retest reliability of the peak knee adduction moment during walking in patients with medial compartment knee osteoarthritis. Arthritis Rheum. 2007;57:1012–1017. doi: 10.1002/art.22899. [DOI] [PubMed] [Google Scholar]
- Creaby MW, Wang Y, Bennell KL, Hinman RS, Metcalf BR, Bowles KA, et al. Dynamic knee loading is related to cartilage defects and tibial plateau bone area in medial knee osteoarthritis. Osteoarthritis Cartilage. 2010;18:1380–1385. doi: 10.1016/j.joca.2010.08.013. [DOI] [PubMed] [Google Scholar]
- Davies-Tuck ML, Wluka AE, Teichtahl AJ, Martel-Pelletier J, Pelletier JP, Jones G, et al. Association between meniscal tears and the peak external knee adduction moment and foot rotation during level walking in postmenopausal women without knee osteoarthritis: a cross-sectional study. Arthritis Res Ther. 2008;10:R58. doi: 10.1186/ar2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gok H, Ergin S, Yavuzer G. Kinetic and kinematic characteristics of gait in patients with medial knee arthrosis. Acta Orthop Scand. 2002;73:647–652. doi: 10.1080/000164702321039606. [DOI] [PubMed] [Google Scholar]
- Holt HL, Katz JN, Reichmann WM, Gerlovin H, Wright EA, Hunter DJ, et al. Forecasting the burden of advanced knee osteoarthritis over a 10-year period in a cohort of 60–64 year-old US adults. Osteoarthritis Cartilage. 2010;19:44–50. doi: 10.1016/j.joca.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurwitz DE, Ryals AB, Case JP, Block JA, Andriacchi TP. The knee adduction moment during gait in subjects with knee osteoarthritis is more closely correlated with static alignment than radiographic disease severity, toe out angle and pain. J Orthop Res. 2002;20:101–107. doi: 10.1016/S0736-0266(01)00081-X. [DOI] [PubMed] [Google Scholar]
- Jackson DW, Simon TM, Aberman HM. Symptomatic articular cartilage degeneration: the impact in the new millennium. Clin Orthop Relat Res. 2001:S14–S25. [PubMed] [Google Scholar]
- Jackson BD, Teichtahl AJ, Morris ME, Wluka AE, Davis SR, Cicuttini FM. The effect of the knee adduction moment on tibial cartilage volume and bone size in healthy women. Rheumatology (Oxford) 2004;43:311–314. doi: 10.1093/rheumatology/keh002. [DOI] [PubMed] [Google Scholar]
- Jiang L, Rong J, Zhang Q, Hu F, Zhang S, Li X, et al. Prevalence and associated factors of knee osteoarthritis in a community-based population in Heilongjiang, Northeast China. Rheumatol Int. 2011 doi: 10.1007/s00296-010-1773-y. (Electronic publication ahead of print) [DOI] [PubMed] [Google Scholar]
- Koo S, Andriacchi TP. A comparison of the influence of global functional loads vs. local contact anatomy on articular cartilage thickness at the knee. J Biomech. 2007;40:2961–2966. doi: 10.1016/j.jbiomech.2007.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence RC, Helmick CG, Arnett FC, Deyo RA, Felson DT, Giannini EH, et al. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum. 1998;41:778–799. doi: 10.1002/1529-0131(199805)41:5<778::AID-ART4>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Li X, Han ET, Newitt D, Majumdar S. T1rho relaxation quantification using spiral imaging: a preliminary study. Conf Proc IEEE Eng Med Biol Soc. 2004;2:1032–1035. doi: 10.1109/IEMBS.2004.1403339. [DOI] [PubMed] [Google Scholar]
- Li X, Benjamin Ma C, Link TM, Castillo DD, Blumenkrantz G, Lozano J, et al. In vivo T(1rho) and T(2) mapping of articular cartilage in osteoarthritis of the knee using 3 T MRI. Osteoarthritis Cartilage. 2007;15:789–797. doi: 10.1016/j.joca.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Pai A, Blumenkrantz G, Carballido-Gamio J, Link T, Ma B, et al. Spatial distribution and relationship of T1rho and T2 relaxation times in knee cartilage with osteoarthritis. Magn Reson Med. 2009;61:1310–1318. doi: 10.1002/mrm.21877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Cheng J, Lin K, Saadat E, Bolbos RI, Jobke B, et al. Quantitative MRI using T(1rho) and T(2) in human osteoarthritic cartilage specimens: correlation with biochemical measurements and histology. Magn Reson Imaging. 2010;29:324–334. doi: 10.1016/j.mri.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luke AC, Stehling C, Stahl R, Li X, Kay T, Takamoto S, et al. High-field magnetic resonance imaging assessment of articular cartilage before and after marathon running: does long-distance running lead to cartilage damage? Am. J Sports Med. 2010;38:2273–2280. doi: 10.1177/0363546510372799. [DOI] [PubMed] [Google Scholar]
- Milner CE, Westlake CG, Tate JJ. Test-retest reliability of knee biomechanics during stop jump landings. J Biomech. 2011;44:1814–1816. doi: 10.1016/j.jbiomech.2011.04.005. [DOI] [PubMed] [Google Scholar]
- Noehren B, Scholz J, Davis I. The effect of real-time gait retraining on hip kinematics, pain and function in subjects with patellofemoral pain syndrome. Br J Sports Med. 2010;45:691–696. doi: 10.1136/bjsm.2009.069112. [DOI] [PubMed] [Google Scholar]
- Pollard CD, Sigward SM, Ota S, Langford K, Powers CM. The influence of in-season injury prevention training on lower-extremity kinematics during landing in female soccer players. Clin J Sport Med. 2006;16:223–227. doi: 10.1097/00042752-200605000-00006. [DOI] [PubMed] [Google Scholar]
- Regatte RR, Akella SV, Lonner JH, Kneeland JB, Reddy R. T1rho relaxation mapping in human osteoarthritis (OA) cartilage: comparison of T1rho with T2. J Magn Reson Imaging. 2006;23:547–553. doi: 10.1002/jmri.20536. [DOI] [PubMed] [Google Scholar]
- Shiomi T, Nishii T, Tanaka H, Yamazaki Y, Murase K, Myoui A, et al. Loading and knee alignment have significant influence on cartilage MRI T2 in porcine knee joints. Osteoarthritis Cartilage. 2010;18:902–908. doi: 10.1016/j.joca.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Snyder KR, Earl JE, O’Connor KM, Ebersole KT. Resistance training is accompanied by increases in hip strength and changes in lower extremity biomechanics during running. Clin Biomech. 2009;24:26–34. doi: 10.1016/j.clinbiomech.2008.09.009. [DOI] [PubMed] [Google Scholar]
- Souza RB, Arya S, Pollard CD, Salem G, Kulig K. Patellar tendinopathy alters the distribution of lower extremity net joint moments during hopping. J Appl Biomech. 2010a;26:249–255. doi: 10.1123/jab.26.3.249. [DOI] [PubMed] [Google Scholar]
- Souza R, Stehling C, Wyman BT, Hellio Le Graverand MP, Li X, Link TM, et al. The effects of acute loading on T1rho and T2 relaxation times of tibiofemoral articular cartilage. Osteoarthritis Cartilage. 2010b;18:1557–1563. doi: 10.1016/j.joca.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Stahl R, Blumenkrantz G, Carballido-Gamio J, Zhao S, Munoz T, Hellio Le Graverand-Gastineau MP, et al. MRI-derived T2 relaxation times and cartilage morphometry of the tibio-femoral joint in subjects with and without osteoarthritis during a 1-year follow-up. Osteoarthritis Cartilage. 2007;15:1225–1234. doi: 10.1016/j.joca.2007.04.018. [DOI] [PubMed] [Google Scholar]
- Stahl R, Luke A, Li X, Carballido-Gamio J, Ma CB, Majumdar S, et al. T1rho, T2 and focal knee cartilage abnormalities in physically active and sedentary healthy subjects versus early OA patients–a 3.0-Tesla MRI study. Eur Radiol. 2009;19:132–143. doi: 10.1007/s00330-008-1107-6. [DOI] [PubMed] [Google Scholar]
- Thorp LE, Sumner DR, Block JA, Moisio KC, Shott S, Wimmer MA. Knee joint loading differs in individuals with mild compared with moderate medial knee osteoarthritis. Arthritis Rheum. 2006;54:3842–3849. doi: 10.1002/art.22247. [DOI] [PubMed] [Google Scholar]
- Vanwanseele B, Eckstein F, Smith RM, Lange AK, Foroughi N, Baker MK, et al. The relationship between knee adduction moment and cartilage and meniscus morphology in women with osteoarthritis. Osteoarthritis Cartilage. 2010;18:894–901. doi: 10.1016/j.joca.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Wheaton AJ, Dodge GR, Borthakur A, Kneeland JB, Schumacher HR, Reddy R. Detection of changes in articular cartilage proteoglycan by T(1rho) magnetic resonance imaging. J Orthop Res. 2005;23:102–108. doi: 10.1016/j.orthres.2004.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y. Relaxation anisotropy in cartilage by NMR microscopy (muMRI) at 14-microm resolution. Magn Reson Med. 1998;39:941–949. doi: 10.1002/mrm.1910390612. [DOI] [PubMed] [Google Scholar]