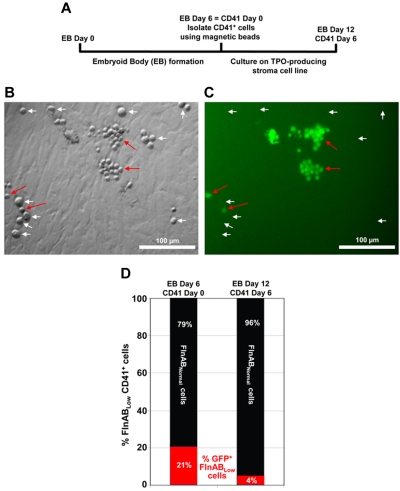
Figure 2.
Filamins are required for efficient megakaryocytopoiesis. (A) Schematic diagram of the protocol used to generate megakaryocytes from cultured ESCs. ESCs were differentiated into EBs using standard procedures. The resulting EBs were cultured for 6 days, at which time CD41-positive cells were selected using magnetic beads. CD41-positive EB-derived cells were then cultured for an additional 6 days in the presence of hTERT-TPO stroma cells, which secrete thrombopoietin and help drive differentiation into specific megakaryocyte precursors. (B-C) DIC and fluorescent images of EB-derived CD41+ cells cultured in the presence of TPO-producing stromal cells at day 4. At this time point, 2 distinct populations of cells exist: large, GFP-negative megakaryocyte precursors that have lost expression of the shRNA that keep filamin levels low (white arrows), and much smaller, GFP-positive cells that retain the filamin A/B shRNA (red arrows). An image was chosen that showed relatively equal numbers of both cell types. However, in the total cell culture, there were many more large, filamin-positive GFP-negative megakaryocyte precursors than there were small, filamin-negative, GFP-positive immature cells (quantitation shown in panel C). (D) Megakaryocyte differentiation in this population was confirmed by GPIbβ antibody staining (data not shown). These data suggest that failure to express filamin results in a delay of megakaryocytopoiesis.