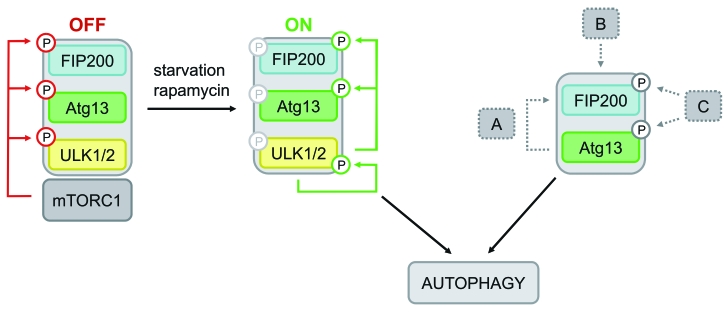
Figure 6.
Model of differential Atg13-dependent autophagy induction pathways. Based on previous findings it has been proposed that mTORC1 associates with the Ulk-Atg13-FIP200 complex under nutrient-rich conditions, phosphorylates Ulk1/2 and Atg13 at inhibitory sites and suppresses Ulk1/2 kinase activity. Following starvation or direct mTORC1 inhibition, this negative regulation is released, Ulk1/2 autophosphorylates itself at activating sites and subsequently phosphorylates both Atg13 and FIP200. This in turn leads to autophagy induction (mTORC1-Ulk1/2-axis). In cells that do respond to mTORC1 inhibition by autophagy induction and do depend on Ulk1 and Ulk2, this pathway is most likely favored. However, the incomparable phenotypes of atg13−/− and ulk1/2−/− DT40 cells let us assume that Atg13 has a more basal function that is not necessarily regulated by Ulk1 or Ulk2, but necessarily requires FIP200 binding capacity. Thus, Atg13 surprisingly has an additional role, besides its proposed function as an adaptor molecule that bridges Ulk1/2 and its substrate FIP200. Several modes of action are conceivable: (A) Atg13 acts in a kinase-independent way, e.g. by stabilizing or recruiting FIP200, (B) the Atg13-FIP200 complex is regulated in a kinase-independent manner, or (C) Atg13-FIP200 is regulated by other kinases than Ulk1/2.