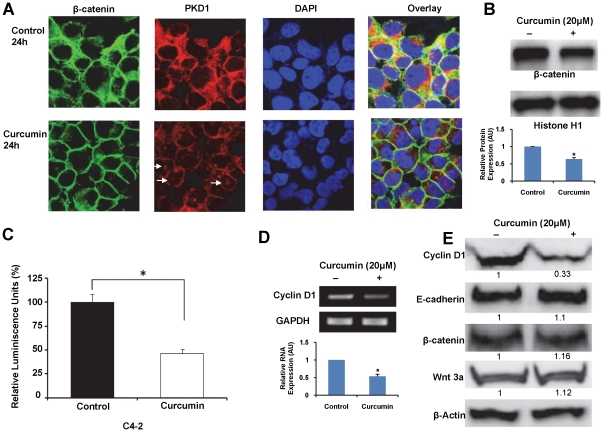
Figure 5. Curcumin inhibits β-catenin transcription activity in prostate cancer cells.
A) Effect of curcumin treatment on the cellular localization of β-catenin and PKD1. C4-2 cells treated with curcumin (20 µM) or DMSO for 24 h were immunostained for β-catenin (green) or PKD1 (red) and counter-stained with DAPI (blue). Curcumin treated cells showed lower cytoplasmic and higher membrane β-catenin staining compared to control cells. In addition, while PKD1 was predominantly localized in the cytoplasm in control cells, curcumin treated cells exhibited staining primarily on the cell membrane and in the nucleus (white arrows), with faint cytoplasmic staining. Original Magnifications 600× with 2× zoom. B) Effect of curcumin on nuclear β-catenin levels. Nuclear proteins isolated from C4-2 cells treated either with curcumin (20 µM) or DMSO were resolved on PVDF membrane and processed for immunoblotting using β-catenin antibody. Histone H1 protein was used as loading control. Densitometric quantitation of β-catenin band intensities, normalized to Histone H1 levels is shown in graph. Curcumin treatment markedly decreased the levels of nuclear β-catenin compared to vehicle treated cells. AU- arbitrary units. C) Effect of curcumin on β-catenin transcription activity in C4-2 prostate cancer cells. The β-catenin transcription activity was measured by transiently transfecting the cells with TCF luciferase reporter construct containing either TCF promoter binding sites (pTOP-FLASH) or mutant TCF promoter binding sites (pFOP-FLASH) along with internal control plasmid containing Renilla luciferase gene (pRL-TK). After 3 h, the cells were treated with curcumin (20 µM) or DMSO for 24 h. The β-catenin transcription activity was first normalized to Renilla luciferase activity, and expressed as a ratio of pTOP-FLASH/pFOP-FLASH activity. The activity of curcumin treated cells was normalized to activity of vehicle treated cells (considered 100%). Curcumin treatment significantly reduced β-catenin transcription activity in C4-2 cells compared to vehicle treated cells. Mean ± SE, n = 3, *p<0.01. D). Effect of curcumin on transcription of cyclin D1. The transcription of cyclin D1 was analyzed from cells treated with curcumin or vehicle control for 24 h. After reverse transcription of RNA to cDNA, PCR amplification of cyclin D1 or internal control GAPDH was carried out using gene specific primers. The amplified products were resolved on 1% agarose gel. The densitometric quantitation of cyclin D1 band intensities normalized to GAPDH levels is shown in graph. Curcumin treatment reduced the expression of cyclin D1. AU- arbitrary units. E). Immunoblot analyses. Cell lysates prepared from curcumin (20 µM) or DMSO treated C4-2 cells were resolved by SDS-PAGE and processed for immunoblotting using specific antibodies. Curcumin treatment markedly decreased cyclin D1 expression, whereas no effect was observed on the expression of total β-catenin, E-cadherin or Wnt 3a. Representative immunoblots from three experiments are shown.