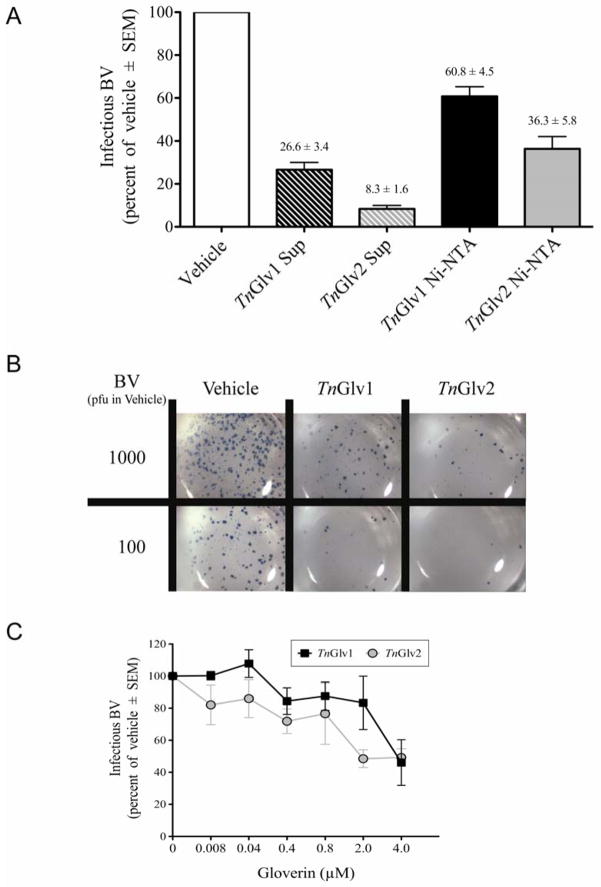
Figure 2. TnGlv1 and TnGlv2 reduced the quantity of infectious AcMNPV-hsp70/lacZ BV.
(A) Infectious BV was reduced when incubated in the presence of Sf9 cell culture supernatants containing TnGlv1 (TnGlv1 Sup), TnGlv2 (TnGlv2 Sup) or Ni-NTA affinity column purified TnGlv1 (TnGlv1 Ni-NTA) or TnGlv2 (TnGlv2 Ni-NTA). The results from replicated plaque assay experiments were normalized to the vehicle treatment (TNM-FH media) for each experiment, which was set to 100 % infectious BV, and the change in the quantity of infectious BV reported as the percent of the vehicle treatment. Means and SEM were calculated from N ≥ 3 replicates per treatment. (B) Plaque assay of Sf9 cells showing blue LACZ-positive plaques of AcMNPV-hsp70/lacZ BV incubated with cell culture supernatants containing TnGlv1, TnGlv2, or vehicle. (C) Concentration dependent antiviral activity of TnGlv1 and TnGlv2 on AcMNPV BV. Plaque assays of AcMNPV-hsp70/lacZ BV incubated with increasing concentrations of affinity column purified TnGlv1 and TnGlv2 protein was correlated with decreasing quantities of infectious BV relative to vehicle treatments (BV incubated with TNM-FH media; N = 4 replicates per treatment).