Abstract
Epithelial ovarian cancer (EOC) is the leading cause of gynecological cancer death in the United States. Cisplatin is a DNA damaging agent initially effective against EOC but limited by resistance. P53 plays a critical role in cellular response to DNA damage and has been implicated in EOC response to platinum chemotherapy. In this study, we examined the role of p53 status in EOC response to a novel combination of cisplatin, sodium arsenite, and hyperthermia. Human EOC cells were treated with cisplatin ± 20μM sodium arsenite at 37°C or 39°C for 1 h. Sodium arsenite ± hyperthermia sensitized wild-type p53-expressing (A2780, A2780/CP70, OVCA 420, OVCA 429, and OVCA 433) EOC cells to cisplatin. Hyperthermia sensitized p53-null SKOV-3 and p53-mutant (OVCA 432 and OVCAR-3) cells to cisplatin. P53 small interfering RNA (siRNA) transfection abrogated sodium arsenite sensitization effect. XPC, a critical DNA damage recognition protein in global genome repair pathway, was induced by cisplatin only in wild-type p53-expressing cells. Cotreatment with sodium arsenite ± hyperthermia attenuated cisplatin-induced XPC in wild-type p53-expressing cells. XPC siRNA transfection sensitized wild-type p53-expressing cells to cisplatin, suggesting that sodium arsenite ± hyperthermia attenuation of XPC is a mechanism by which wild-type p53-expressing cells are sensitized to cisplatin. Hyperthermia ± sodium arsenite enhanced cellular and DNA accumulation of platinum in wild-type p53-expressing cells. Only hyperthermia enhanced platinum accumulation in p53-null cells. In conclusion, sodium arsenite ± hyperthermia sensitizes wild-type p53-expressing EOC cells to cisplatin by suppressing DNA repair protein XPC and increasing cellular and DNA platinum accumulation.
Keywords: ovarian cancer, sodium arsenite, cisplatin, hyperthermia, XPC, p53
Epithelial ovarian cancer (EOC) is the leading cause of gynecological cancer death among women in the United States (Jemal et al., 2010). Front-line treatment is cytoreductive surgery followed by intraperitoneal and/or intravenous platinum chemotherapy with taxane (McGuire et al., 1996). Within 5 years after initial treatment, the disease recurs in 60–70% of patients. In addition, ∼25% of ovarian cancers are “innately” resistant to platinum and respond poorly to initial chemotherapy.
Cisplatin and its analogues cause DNA damage to induce cell death (Stewart, 2007). However, cellular processes such as enhanced platinum-DNA damage tolerance, platinum-DNA repair, cisplatin metabolism, and cellular export and reduced accumulation confer resistance to cisplatin (Cepeda et al., 2007). Resistance decreases the effectiveness of cisplatin. Therefore, it is very important to develop pharmacological agents to improve cisplatin efficacy and decrease morbidity and mortality.
Combined hyperthermia and cisplatin are used to treat ovarian cancer (Helm et al., 2008). However, complete remission is not attained (Dovern et al., 2010). The goal of this study was to determine if adding sodium arsenite to combined cisplatin and hyperthermia will sensitize ovarian cancer cells to cisplatin.
Arsenic is both an environmental hazard and a chemotherapeutic. Trisenox (arsenic trioxide) was approved by the U.S. Food and Drug Administration in 2001 for the treatment of “all trans” retinoic acid-resistant acute promyelocytic leukemia (Cohen et al., 2001). In vitro studies demonstrate that arsenic trioxide induces apoptosis in solid cancer cells including gastric, colon, pancreatic, lung, prostate, and ovarian cancer (Cui et al., 2008; Murgo, 2001). Arsenic trioxide inhibits the growth of orthotopic metastatic prostate cancer and peritoneal metastatic ovarian cancer (Maeda et al., 2001; Zhang and Wang, 2006). Arsenic sensitizes cancer cells to hyperthermia, radiation, cisplatin, adriamycin, doxorubicin, and etoposide (Chun et al., 2002; Griffin et al., 2003; Uslu et al., 2000; Wang et al., 2001). Arsenic is additive or synergistic with cisplatin in inducing cytotoxicity following prolonged exposure (Wang et al., 2001; Zhang et al., 2009). Mechanisms of arsenic-induced cell death include formation of oxidative DNA damage (Nakagawa et al., 2002), activation of the Fas pathway (Kong et al., 2005), inhibition of nucleotide excision repair (NER) pathway (Muenyi et al., 2011; Nollen et al., 2009), and causation of mitotic catastrophe (Taylor et al., 2008).
Arsenic has biological effects similar to cisplatin and hyperthermia. Like cisplatin, arsenic is detoxified by glutathionylation and exported by multidrug resistant proteins (Leslie et al., 2004). Like hyperthermia, arsenic causes oxidative stress and mitotic catastrophe (Taylor et al., 2008). Thus, arsenic potentially can effectively augment both hyperthermia- and cisplatin-induced cytotoxicity.
In response to DNA damage, p53 is activated and stabilized by upstream DNA damage sensors. Activated p53 regulates cell cycle arrest, DNA repair, and apoptosis (Efeyan and Serrano, 2007). The p53 gene is mutated in 50% of human cancers (Olivier et al., 2002), including epithelial ovarian cancer (Schuijer and Berns, 2003). The role of p53 in ovarian cancer response to platinum chemotherapy remains unclear. Several clinical studies suggest better response to platinum chemotherapy in patients with p53-mutated tumors than those with wild-type p53 tumors (Havrilesky et al., 1995; Nakayama et al., 2003; Okuda et al., 2003). Likewise, in vitro studies also demonstrate that p53-mutated and p53-null cancer cells are more sensitive to cisplatin than those expressing wild-type p53 (Hagopian et al., 1999; Havrilesky et al., 1995). Therefore, presence of wild-type p53 is generally associated with resistance to cisplatin.
Enhanced platinum-DNA damage repair by the NER pathway is an important mechanism of cisplatin resistance (Parker et al., 1991). P53 regulates NER by transcriptionally regulating xeroderma pigmentosum group C protein (XPC) and DNA damage binding protein 2 (DDB2), which are DNA damage recognition proteins in global genome repair-NER subpathway (Ford, 2005). XPC is required for platinum-DNA damage repair (Neher et al., 2010). We recently showed that sodium arsenite and hyperthermia interfere with mechanisms of cisplatin resistance in metastatic tumors by attenuating XPC expression and enhancing platinum accumulation (Muenyi et al., 2011).
The present manuscript addresses how the p53 status of ovarian cancer cells affects response to the combination of cisplatin, sodium arsenite, and hyperthermia. We show here that combined sodium arsenite and hyperthermia sensitize wild-type p53-expressing ovarian cancer cells to cisplatin by attenuation of cisplatin-induced XPC and enhancement of cellular and DNA platinum accumulation.
MATERIALS AND METHODS
Chemicals.
Bovine serum albumin (BSA), MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide), RNase A, cisplatin, and sodium arsenite were purchased from Sigma-Aldrich (St Louis, MO). Dimethyl sulfoxide (DMSO) was purchased from Fisher Scientific (Pittsburgh, PA). Stock solutions (cisplatin in PBS [1 mg/ml] and sodium arsenite in water [10mM]) were prepared freshly on the day of treatment and filter sterilized (0.22 μm) prior to use. Trisenox (arsenic trioxide dissolved in 1M NaOH) and sodium arsenite both generate the same reactive species (As[OH]3) in solution (pharmacological form of arsenic). We used sodium arsenite for our studies because it is readily soluble in water and cell culture media. The concentration of arsenite we used corresponds to the level that could be incorporated into an intraperitoneal chemotherapeutic regimen in ovarian cancer patients similar to that used in our published mouse model studies (Muenyi et al., 2011). This concentration was based on calculation of the total dose of arsenic trioxide given intravenously to an acute promyelogenous leukemia patient in a single day, as if it was put in 2-l saline solution to be applied intraperitoneally. Treatment of cells with arsenite alone for 1 h had no impact on cell viability judged in MTT or colony forming assays (data not shown).
Cells and cell culture.
A2780 and A2780/CP70 human ovarian cancer cells were the kind gifts of Dr Eddie Reed (The Mitchell Cancer Institute, University of South Alabama, Mobile, AL). SKOV-3 human ovarian cancer cells were the kind gift of Dr Donald Miller (Department of Medicine, University of Louisville, Louisville, KY). OVCA 420, OVCA 429, OVCA 432, and OVCA 433 human ovarian cancer cells were the kind gift of Dr Zahid Siddik (Department of Gynecologic Oncology, The University of Texas M.D. Anderson Cancer Center, Houston, Texas). OVCAR-3 human ovarian cancer cells were purchased from American Type Culture Collection (Manassas, VA). A2780, A2780/CP70, OVCA 420, OVCA 429, OVCA 432, and OVCA 433 cells were maintained in RPMI 1640 media supplemented with 10% fetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin, 2mM L-glutamine, and 0.2 units/ml insulin (Novolog, Novo Nordisk Inc., Princeton, NJ). SKOV-3 cells were maintained in McCoy’s 5A media supplemented 10% FBS and 100 U/ml penicillin, 100 μg/ml streptomycin. OVCAR-3 cells were maintained in RPMI 1640 media supplemented with 20% fetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin, 2mM L-glutamine and 0.01 mg/ml bovine insulin (Sigma). Cells were cultured in an atmosphere of 95% humidity and 5% CO2 at 37°C. Cells were passaged twice weekly and replated at a density of 1 × 106 cells/150 mm dish.
Cell viability assay.
The growth inhibitory effects of cisplatin, sodium arsenite, and hyperthermia were evaluated using MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) cell viability assay. Briefly, 2500 cells per well were seeded overnight in a 96-well plate. Cells were treated with cisplatin ± 20μM sodium arsenite at 37°C or 39°C for 1 h. After treatment, cells were washed twice with PBS and refed with drug-free medium and incubated at 37°C for 5 days prior to MTT assay. Control for no surviving cells (blank) was cells treated with 0.1 mg/ml hygromycin B (Mediatech, Herndon, VA). Five days after treatment, cell culture media were dumped from the cells and the dish blotted on Whatman paper, 25 μl of 2 mg/ml MTT (in PBS) was added per well and the dish incubated at 37°C for 1 h. Then 200 μl DMSO was added per well and the dish incubated at 37°C for 1 h. Absorbance was measured at 570 nm. The absorbance values corresponded to the number of viable cells. Cell viability was calculated as follows and plotted against concentration of cisplatin.
Data are expressed as means ± SEM of at least four independent experiments. Each experiment was done in triplicate wells for each treatment condition.
The results of MTT assays corresponded to results obtained in colony forming assays performed by replating cells at low density immediately after treatment with cisplatin (data not shown).
P53 and XPC siRNA transfections.
One million cells were transfected with 0.4μM of either XPC or p53 smart pool small interfering RNAs (siRNAs) (Dharmacon, no. L-016040-00 and M-003329-01, respectively), nontargeting control (NSC) pool (Dharmacon, no. D-001206-13-05), or 1x universal buffer (Dharmacon, no. B-001050-UB-015) using the Amaxa Nucleofector Kit V (Lonza, cat no. VCA-1003, 2.5 ml). After transfection, 2500 cells per well were plated in 96-well plate for MTT assay and 1 × 105 cells were plated in 6 cm dish for Western blot analysis. Cells were incubated at 37°C for 23 h and then treated with cisplatin ± 20μM sodium arsenite at 37°C for 1 h. After treatment, cells were washed twice with PBS and refed with drug-free medium and incubated at 37°C for 5 days prior to MTT assay. Protein lysates were collected at 0 (immediately) and 24 h after treatment for Western blot analyses.
Western blot analyses.
Total cellular lysates were prepared from treated cells at 0 (immediately), 6, 12, 24, and 36 h after treatment. Cells were lysed with lysis solution (10mM Tris-HCl pH 7.4, 1mM EDTA, 1% sodium dodecyl sulfate, 180 μg/ml phenylmethylsulphonylfluoride). After removal of debris by centrifugation at 13,000 × g for 45 min at 4°C, total protein concentration in supernatant was determined by Bradford assay (Bio-Rad, Hercules, CA), using BSA as standard. Proteins were loaded (30–40 μg/lane) and resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and electro-transferred to nitrocellulose membranes. Membranes were probed with mouse monoclonal antibodies for p53 (Neomarkers, DO-1, dilution 1: 1000), XPC (Abcam, no. ab6264, dilution 1:1000), ß-actin (Sigma, no. A 5441, dilution 1:10,000), or rabbit polyclonal antibodies for phospho-p53 Ser15 (Cell Signaling Technology, no. 9284, dilution 1:500), XPC (Novus, no. NB100-58801, dilution 1:10,000), and XPC (Santa Cruz, A-5, no. SC-74411, 1:500). Secondary antibodies (rabbit anti-mouse IgG, no. 81-6120 or goat anti-rabbit, no. 81-6120, dilution 1:2500) conjugated to horseradish peroxidase (Zymed Laboratories, Inc., South San Francisco, CA) were bound to primary antibodies, and protein bands detected using enhanced chemiluminescence substrate (Pierce, Rockford, IL) followed by exposure to Kodak XAR x-ray film. ß-actin was used as the loading control.
Cellular platinum accumulation studies.
Cells (1 × 106/10 cm dish) were treated with cisplatin ± 20μM sodium arsenite at 37°C or 39°C for 1 h (designated CP 37, CPA 37, CP 39, CPA 39). The cell monolayers were washed twice with PBS, harvested, and lysed with protein lysis solution. Samples were removed for protein determination using the bicinchoninic acid method according to manufacturer’s instructions (Pierce, Rockford, IL, microwell plate protocol). Samples were prepared and assayed for platinum using inductively coupled plasma mass spectrometry (ICP-MS) as previously described (Muenyi et al., 2011). Cellular platinum levels were expressed as nanogram platinum per milligram protein. Results are the mean of 3 ICP-MS determinations for each data point ± SD from three independent experiments.
Determination of DNA bound platinum.
After treatment, cells were lysed with DNA lysis buffer (0.5M Tris-HCl (pH 8.0), 20mM EDTA, 10mM NaCl, 1% SDS, and 0.5 mg/ml proteinase K) and incubated overnight at 37°C. Residual proteins were precipitated using saturated NaCl solution followed by centrifugation (room temperature, 30 min, 500 ×g). The supernatants were collected, mixed with two volumes of 96% ethanol, and inverted several times to precipitate DNA. The precipitated DNA was recovered and incubated at 37°C with 100-μg heat-treated RNase A/ml 1X TE buffer (10mM Tris-HCl [pH 7.4] and 1.0mM Na2EDTA) for 3 h. DNA was recovered using 11M ammonium acetate pH 6.5 and two volumes of cold 96% ethanol. DNA was quantified by A260 and purity determined by A260/A280 ratio. Samples were prepared and assayed for platinum using ICP-MS as previously described (Muenyi et al., 2011). Platinum bound to DNA was expressed as picogram platinum per microgram DNA. Results are the mean of 3 ICP-MS determinations for each data point ± SD from three independent experiments.
Statistical analysis.
An analysis of covariance model was fitted to data from the cell viability experiments, regressing the log of the percent viability onto a quadratic function of the cisplatin concentration. The full model, with separate regression curves for each experimental combination, was
| (1) |
where i = 1 (37°C), 2 (39°C) indicates the temperature setting, j = 1, 2 indicates the presence/absence of sodium arsenite, k = 1, . . , K indicates the cisplatin level, and l = 1, . . , N indicates the replicate number (N = 3 or 4 in all cases). The response yijkl is the percent viability, the parameters αij, β1ij, and β2ij represent the intercept, slope, and quadratic term, respectively, for the ijth combination of temperature setting and arsenite treatment, and ϵijkl ∼ N(0, σ2) is a normally distributed error term. In all, the model contains 16 parameters that are estimated. To test for interaction between the temperature setting and presence/absence of sodium arsenite, this model was compared with a reduced model which had only main effect terms for the two experimental factors. This model was parameterized as
| (2) |
with the constraint α11 = α21 = β11 = β21 = γ11 = γ21 = 0 for identifiability purposes. This model contains 12 total parameters, and an F-test comparing the residual sums of squares between the two models was used to test for significance of the interaction terms. To test for overall significance of the experimental factors, Model 1 was compared with a reduced model with only three parameters, an intercept plus linear, and quadratic terms for cisplatin. To test for differences in regression curves between experimental groups, e.g., CPA 39°C versus CPA 37°C, Model 1 was compared with a reduced model obtained under the restriction of the null hypothesis (e.g., that the regression curves for CPA 39°C and CPA 37°C are the same). Main effects for the two experimental conditions, temperature and arsenite treatment, were obtained by comparing Model 2 with a reduced model obtained by removing the parameters associated with that experimental condition. Similar tests were performed for the other cell viability experiments and for the platinum accumulation experiments. Additional statistical analyses were performed using one-way analysis of variance and Student’s t-test, where noted. Model fitting and testing were carried out using the R statistical programming software, version 2.12.1 (R Development Core Team, 2011). An α level of 0.05 was used to determine statistical significance, and all experiments were performed using at least three independent biological replicates.
RESULTS
Sodium Arsenite ± Hyperthermia Selectively Sensitizes Wild-Type p53-Expressing Ovarian Cancer Cells to Cisplatin
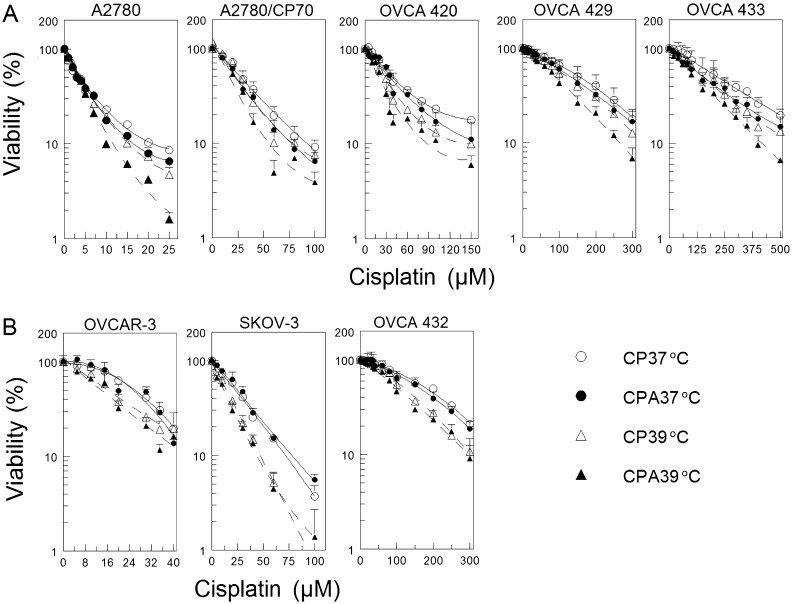
P53 regulates DNA repair and is frequently mutated in ovarian cancer cells. Thus, it is very important to determine if the p53 status will affect response to DNA damaging agent cisplatin combined with sodium arsenite and hyperthermia. We used wild-type p53-expressing cisplatin-sensitive (A2780) and cisplatin-resistant (A2780/CP70, OVCA 420, OVCA 429, and OVCA 433) and p53-null (SKOV-3) and p53-mutated (OVCAR-3 and OVCA 432) cisplatin-resistant human ovarian cancer cells for this study. Our results show that cotreatment with sodium arsenite or hyperthermia moderately enhanced cisplatin cytotoxicity in cells expressing wild-type p53 (Fig. 1A). Overall tests of treatment effect were all highly significant (p < 10−4, see Supplementary table 1), whereas tests of interaction between hyperthermia and sodium arsenite treatment were only significant for the A2780 and OVCA 429 cell lines (p < 10−4). Differences between 37°C and 39°C temperatures were significant both as main effects and when evaluated separately in CPA and CP treatments (p < 10−4 for all cell lines, Supplementary table 1). The effect of arsenite treatment was also significant as a main effect and when evaluated separately within each temperature, with the exception of CPA 37°C versus CP 37°C for the OVCA 429 cell line. However, combined sodium arsenite and hyperthermia more effectively potentiates cisplatin cytotoxicity in wild-type p53-expressing cells (Fig. 1A, CPA 39°C, and Supplementary table 1). In contrast, hyperthermia clearly sensitized p53-null and p53-mutated cells to cisplatin with or without arsenite cotreatment but addition of arsenite only marginally sensitized these cells (Fig. 1B). Again, overall tests of treatment effect were highly significant (p < 10−4). However, tests for interaction between hyperthermia and sodium arsenite treatment were all nonsignificant. Hyperthermia had a pronounced effect in both CP- and CPA-treated cell lines, but arsenite treatment was only significant for 39°C samples (with the exception of OVCA 432, which had p = 0.04 for CPA 37°C vs. CP 37°C). Combining sodium arsenite with hyperthermia did increase cisplatin sensitivity in cells lacking functional p53 to some extent (Fig. 1B and Supplementary table 1).
FIG. 1.
Cell viability as determined by MTT assay. (A) Wild-type p53-expressing A2780, A2780/CP70, OVCA 420, OVCA 429, and OVCA 433 cells. (B) P53-null (SKOV-3) and p53-mutated (OVCAR-3 and OVCA 432) cells. Cells were cotreated with the indicated concentrations of cisplatin with (filled symbols) or without (open symbols) 20μM sodium arsenite and incubated at 37°C (circles) or 39°C (triangles) for 1 h. Cells were then washed twice with PBS and refed with fresh media and incubated for 5 days at 37°C. MTT assay was performed on day 5. Data are expressed as percentage of untreated control and plotted as means ± SEM of at least four independent experiments each performed with triplicate wells. R2 values for the best fitting quadratic polynomial curves were all > 0.99.
Sodium Arsenite Requires Functional p53 to Sensitize Cells to Cisplatin
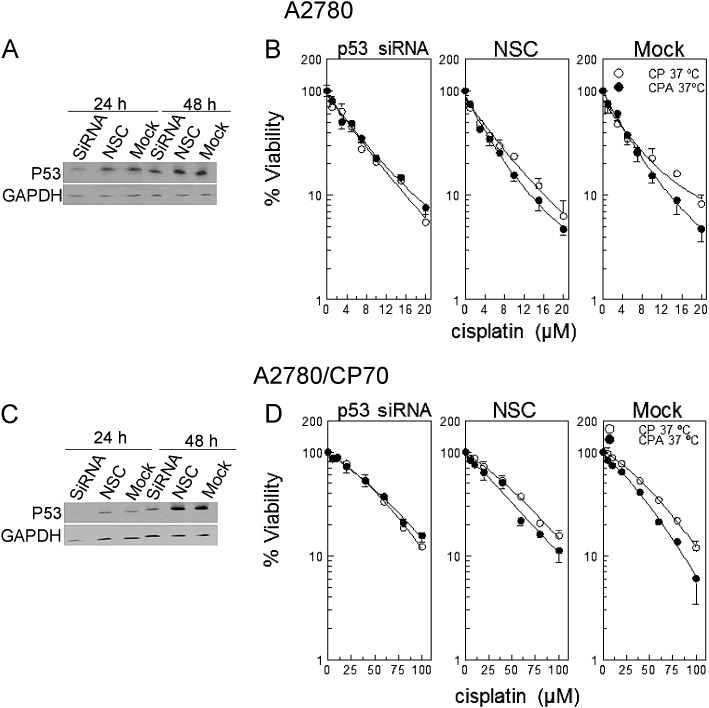
Data in Figure 1 indicate that sodium arsenite sensitizes primarily wild-type p53-expressing cells to cisplatin. To test whether sodium arsenite requires functional p53 to sensitize cells to cisplatin, we transfected A2780 and A2780/CP70 cells with p53 smart pool siRNA, NSC, or universal buffer (mock). Western blot data confirmed suppression of p53 at 24 h after transfection for A2780 cells (Fig. 2A) and at 24 and 48 h for A2780/CP70 cells (Fig. 2C). Results of viability determinations indicate that suppression of p53 abrogates sodium arsenite sensitization to cisplatin (Figs. 2B and D, p > 0.5 for differences between CPA and CP curves, see Supplementary table 2). Moderate sensitization to cisplatin by cotreatment with sodium arsenite was observed in cells transfected with NSC and mock (Figs. 2B and D, p < 10−4 except NSC in A2780 cells, Supplementary table 2). These data indicate that sodium arsenite requires functional p53 to sensitize cells to cisplatin.
FIG. 2.
Cell viability and Western blot analysis of cells transfected with p53 siRNA. (A and C) Western blot analyses of A2780 and A2780/CP70 cells respectively each transfected with p53 siRNA, NSC, or mock. (B and D) Viability of respective A2780 and A2780/CP70 cells transfected with p53 siRNA, NSC, or mock. A2780 and A2780/CP70 cells were transfected with p53 smart pool siRNA, NSC, or mock. At 23 h after transfection, cells were treated with the indicated concentrations of cisplatin with (•) or without (o) 20μM sodium arsenite for 1 h at 37°C. Protein lysates for Western blot analyses were prepared at 24 and 48 h after transfection. MTT assay was performed 5 days after treatment. Data in panels A and C are representative of three independent experiments. Data in panels B and D are means ± SD from three independent experiments.
Induction of XPC Is p53-Dependent
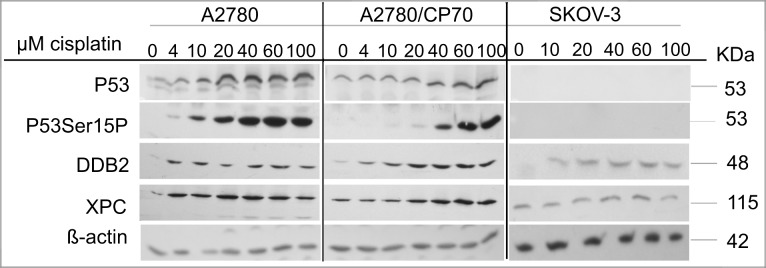
Cisplatin-DNA damage is repaired by the NER system. P53 regulates the NER pathway by transcriptionally activating XPC and DDB2, which are DNA damage recognition proteins in the global genome repair-NER subpathway (Ford, 2005). We recently showed that sodium arsenite and hyperthermia suppress cisplatin-induced XPC in metastatic EOC xenograft tumors (Muenyi et al., 2011). Thus, the observed p53 dependence of sodium arsenite sensitization may be related to the role of p53 in DNA repair. We determined the expression of p53, p53 phosphorylated on ser15 (p53Ser15P), DDB2, and XPC in A2780, A2780/CP70, and SKOV-3 cells 24 h after treatment (Fig. 3). P53 and p53Ser15P induction occurred in a cisplatin concentration–dependent manner in the wild-type p53-expressing A2780 and A2780/CP70 cells (Fig. 3). DDB2 induction in response to cisplatin treatment was robust in the wild-type p53-expressing cells but modest in p53-null SKOV-3 cells. Induction of XPC occurred only in wild-type p53-expressing A2780 and A2780/CP70 cells. Similarly, XPC induction was observed also in wild-type p53-expressing OVCA 420 cells (data not shown). These data suggest that XPC induction by cisplatin is mediated by p53 in these EOC cell lines.
FIG. 3.
Cisplatin concentration–dependent induction of p53, p53Ser15P, XPC, and DDB2. Cells were treated with the indicated concentrations of cisplatin (CP) at 37°C for 1 h. After treatment, cells were washed twice with PBS and incubated in drug-free media for 24 h, and protein lysates were then prepared and analyzed by Western blot as detailed in the Materials and Methods section. ß-actin is the loading control. Blots are representative of three independent experiments.
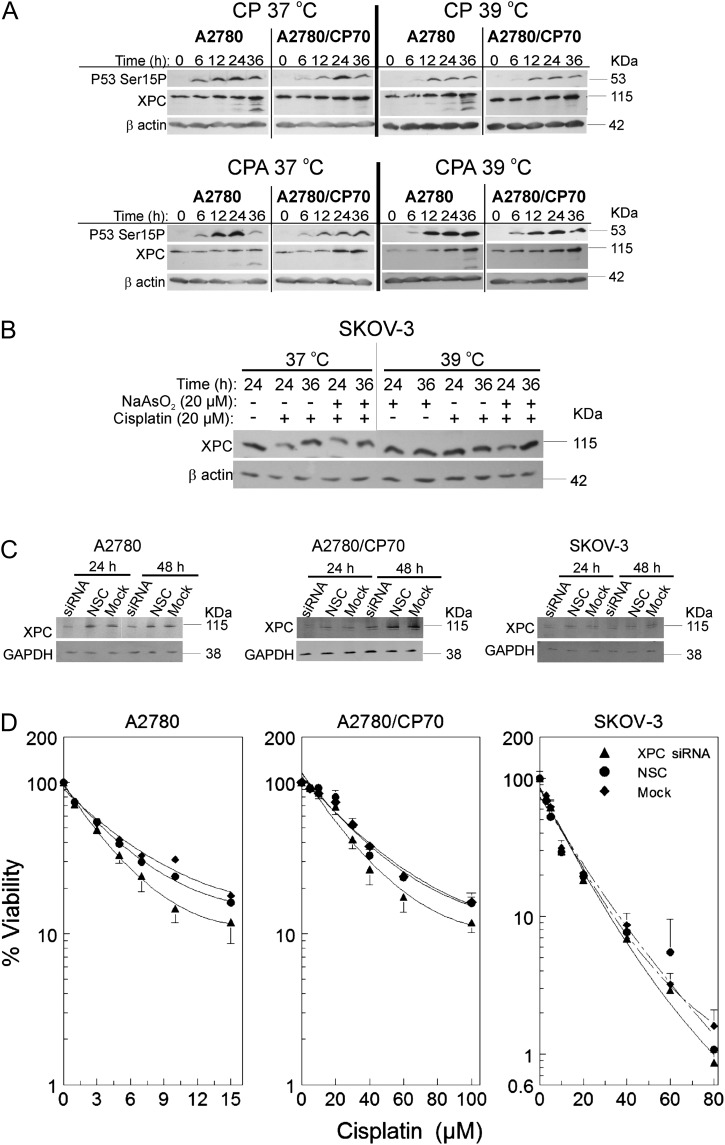
Sodium Arsenite ± Hyperthermia Sensitizes Wild-Type p53-Expressing Cells to Cisplatin by Attenuating XPC
Data in Figure 3 indicate that induction of XPC occurs only in wild-type p53-expressing cells. Therefore, we determined if sodium arsenite and/or hyperthermia alter XPC levels. Cells were treated for 1 h with their respective half maximal inhibitory concentration (IC50) cisplatin ± 20μM sodium arsenite at 37°C or 39°C. P53 Ser15P, and XPC were induced in a time-dependent manner after cisplatin treatment in A2780 and A2780/CP70 cells (Fig. 4A, CP 37°C). Cotreatment with sodium arsenite attenuated cisplatin-induced XPC in both A2780 and A2780/CP70 cells (Fig. 4A, CPA 37°C). Hyperthermia cotreatment did not alter cisplatin-induced XPC (Fig. 4A, CP 39°C). Combined treatment with sodium arsenite and hyperthermia also attenuated cisplatin-induced XPC (Fig. 4A, CPA 39°C). XPC was not induced by cisplatin in p53-null SKOV-3 cells and sodium arsenite ± hyperthermia did not suppress XPC in these cells (Fig. 4B). In order to better understand the significance of XPC suppression, we transfected A2780, A2780/CP70, and SKOV-3 cells with XPC smart pool siRNA, NSC, or mock. Suppression of XPC was confirmed by Western blot (Fig. 4C). Suppression of XPC moderately sensitized A2780 and A2780/CP70 cells to cisplatin (p < 10−4 vs. NSC and Mock, Supplementary table 3) but had no effect on SKOV-3 cells (Fig. 4D), similar to the effects observed for arsenite cotreatment (Fig. 1). These data suggest that attenuation of XPC by sodium arsenite ± hyperthermia is a mechanism of sensitizing wild-type p53-expressing EOC cells to cisplatin.
FIG. 4.
Suppression of cisplatin-induced XPC by sodium arsenite and hyperthermia and the effect of XPC siRNA transfection on cisplatin cytotoxicity. (A and B) Western blot analyses of XPC and p53Ser15P in A2780 and A2780/CP70 cells. Cells were treated with their IC50 cisplatin (CP; A2780, 4μM; A2780/CP70, 40μM; SKOV-3, 20μM) or CP plus 20μM sodium arsenite (CPA) at 37 or 39°C for 1 h. After treatment, cells were washed with PBS and incubated in drug-free media. Protein lysates were prepared at 0, 6, 12, 24, and 36 h for Western blot analyses. ß-actin is the loading control. Data are representative blots of three independent experiments. (B) Western blot analyses of XPC in SKOV-3 cells. Cells were treated with cisplatin ± sodium arsenite as indicated for 1 h, then washed and reincubated in drug-free media for 24 or 36 h. Then, lysates were prepared for Western blot analyses. (C) Western blot analyses of XPC after XPC siRNA transfection. (D) Viability of A2780, A2780/CP70, and SKOV-3 cells after XPC siRNA (▴), NSC (•), or mock (♦) transfection. Cells were transfected with XPC smart pool siRNA, NSC, or mock. At 23 h after transfection, cells were treated with the indicated concentrations of cisplatin (D) or respective IC50 cisplatin (CP; A2780, 4μM; A2780/CP70, 40μM; SKOV-3, 20μM) (C). After treatment, cells were washed twice with PBS and incubated in drug-free media. MTT assay was performed 5 days after treatment. Protein lysates for Western blot analyses were prepared at 24 and 48 h after transfection. Data in panels A, B, and C are representative blots for three independent experiments. Data in panel D are plotted as means ± SD of three independent experiments.
Hyperthermia ± Sodium Arsenite Enhance Cellular Accumulation of Cisplatin
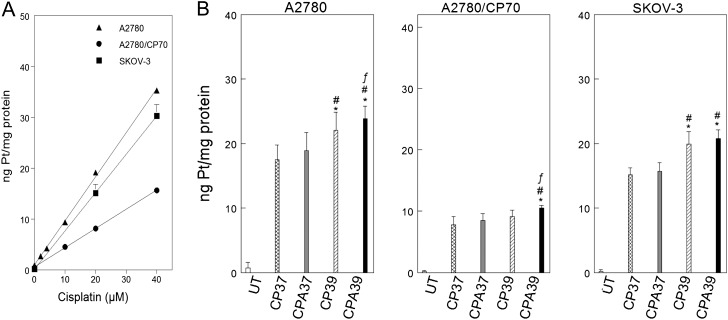
Decreased cellular accumulation is an important mechanism of cisplatin-resistance (Cepeda et al., 2007; Jones et al., 1991). We used ICP-MS to determine cellular levels of platinum to see if differential cellular platinum accumulation contributes to resistance and also to determine if hyperthermia and/or sodium arsenite enhance platinum accumulation (Fig. 5). Platinum accumulation linearly correlated with cisplatin concentration (Fig. 5A). A2780 and SKOV-3 cells accumulated similar levels of platinum (p = 0.97, Supplementary table 4) and approximately twofold more platinum than A2780/CP70 cells (p < 10−4, Fig. 5A and Supplementary table 4). Hyperthermia alone or in combination with sodium arsenite enhanced platinum accumulation in A2780 cells (Fig. 5B, left panel). Cotreatment with sodium arsenite and hyperthermia significantly enhanced cellular platinum accumulation in A2780/CP70 cells (Fig. 5B, center panel). In contrast, only hyperthermia enhanced cellular accumulation of platinum in SKOV-3 cells (Fig. 5B, right panel).
FIG. 5.
Cellular platinum accumulation and effect of sodium arsenite and hyperthermia. (A) Cisplatin concentration–dependent accumulation of platinum in A2780 (▴), A2780/CP70 (▪), and SKOV-3 (•) cells. The regression equation for A2780 cells is: y = 0.68 + 0.74x; A2780/CP70 cells, y = 0.55 + 0.30x; SKOV-3 cells, y = 0.11 + 0.75x; R2 = 0.95 for the overall fit to the data. (B) Effect of sodium arsenite and hyperthermia on cellular platinum accumulation. A2780, A2780/CP70, and SKOV-3 cells were treated with the indicated cisplatin concentrations for 1 h at 37°C (A) or with 40μM cisplatin ± 20μM sodium arsenite at 37°C or 39°C for 1 h (B). Cells were harvested immediately after treatment for total cellular platinum determination. Data are plotted as means ± SD of three independent experiments. p < 0.05. *compared with CP37, # compared with CPA 37, and f compared with CP39.
Hyperthermia ± Sodium Arsenite Increase Platinum Binding to DNA
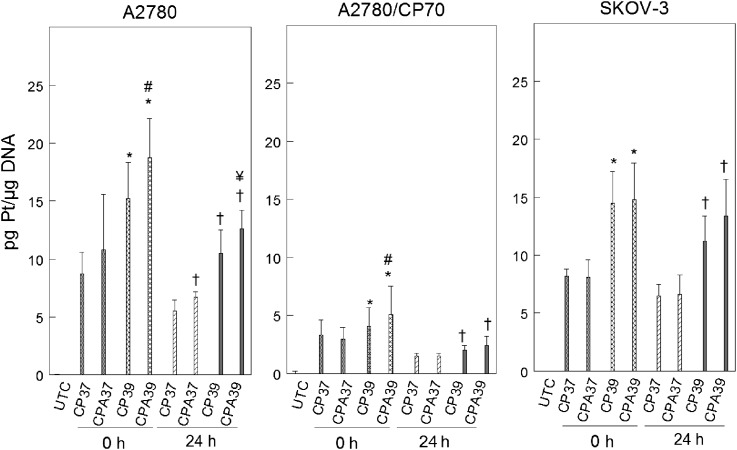
The cytotoxicity of cisplatin is known to depend on its direct interaction with DNA to form bulky platinum-DNA adducts (Earley and Turchi, 2011). We tested the hypothesis that sodium arsenite and hyperthermia sensitize cells to cisplatin by increasing platinum binding to DNA. Using ICP-MS, we determined platinum bound to DNA immediately after treatment (0 h) to measure initial platinum binding to DNA and 24 h after treatment to determine repair of platinum-DNA adducts. We observed that hyperthermia alone or in combination with sodium arsenite significantly increased initial platinum binding to DNA in A2780 and A2780/CP70 cells (Fig. 6). In SKOV-3 cells, only hyperthermia favored initial binding (0 h). Platinum retention on DNA 24 h after treatment was favored by sodium arsenite, hyperthermia, or the combination in A2780 cells. Platinum retention in A2780/CP70 and SKOV-3 cells was favored by hyperthermia only.
FIG. 6.
Alteration of platinum bound to DNA by sodium arsenite and hyperthermia. A2780, A2780/CP70, and SKOV-3 cells were treated with 40μM cisplatin for 1 h at 37°C or 39°C. Total genomic DNA was isolated at 0 (immediately) and 24 h after treatment for platinum bound to DNA determination. Data are means ± SD of three independent experiments. p < 0.05; *compared with CP37 0 h, # compared with CP39 0 h, † compared with CP37 24 h, and ¥ compared with CP39 24 h.
DISCUSSION
This study was aimed at determining the efficacy and mechanisms of action of a novel combination of cisplatin, sodium arsenite, and hyperthermia in human EOC cells with different p53 status. We used a panel of wild-type p53-expressing cisplatin-sensitive and cisplatin-resistant, as well as, p53-null and p53-mutated cisplatin-resistant human EOC cells for this study. We showed for the first time that combining sodium arsenite and hyperthermia sensitizes wild-type p53-expressing cells to cisplatin (Fig. 1A). In contrast, only hyperthermia sensitized p53-null or p53-mutated cells to cisplatin (Fig. 1B). Knockdown of p53 abrogated sodium arsenite sensitization to cisplatin-induced cytotoxicity (Fig. 2). Taken together, these results indicate that the sodium arsenite effect is p53-dependent and the hyperthermia effect is not.
Cisplatin cytotoxicity involves platinum-DNA damage formation. However, enhanced platinum-DNA damage repair confers resistance to cisplatin. Platinum-DNA damage is repaired by the NER pathway (Martin et al., 2008). P53 regulates NER by transcriptionally regulating XPC and DDB2, critical DNA damage recognition proteins in global genome repair, a subpathway of NER (GGR-NER) (Ford, 2005). XPC is required for platinum-DNA damage recognition by GGR-NER (Neher et al., 2010). Basal expression of XPC was observed in both wild-type p53 and p53-null cells, consistent with a report that in addition to p53, other proteins are involved in regulating XPC levels (Lin et al., 2009). However, cisplatin-dependent induction of XPC occurred only in wild-type p53-expressing cells but not in p53-null cells (Fig. 3), indicating that cisplatin-induction of XPC is regulated by p53 in these cells. Cotreatment with sodium arsenite alone or in combination with hyperthermia suppressed cisplatin-induced XPC in the wild-type p53 cells (Fig. 4A, CPA 37°C and CPA 39°C), consistent with our in vivo findings (Muenyi et al., 2011). Suppression of XPC by sodium arsenite ± hyperthermia is a mechanism of sensitizing wild-type p53-expressing cells to cisplatin because XPC siRNA transfection moderately sensitized A2780 and A2780/CP70 cells to cisplatin (Fig. 4E). Given that XPC is critical for platinum-DNA damage recognition in GGR-NER, attenuation of XPC expression could diminish the assembly of the downstream NER repair complex (Nollen et al., 2009) and subsequently decrease DNA repair. In addition, suppression of XPC by arsenite may enhance oxidative DNA damage (Liu et al., 2010) thereby augmenting cisplatin cytotoxicity. Because induction of XPC by cisplatin is dependent on p53 (Supplemental figure 1) and because arsenite selectively suppressed cisplatin-induced XPC only in cells expressing functional p53, arsenite is possibly inhibiting p53 function directly or indirectly in order to abrogate XPC expression. Arsenic has been reported to inhibit p53 DNA–binding activity by inhibiting casein kinase 2, which is required for p53 phosphorylation on serine 392 (p53Ser392P) (Tang et al., 2006). However, p53Ser392P levels were unaffected by sodium arsenite and/or hyperthermia in A2780, A2780/CP70, and OVCA 420 cells (data not shown), suggesting that arsenite is not acting by inhibiting casein kinase 2 (CK2) in these ovarian cancer cells. Therefore, other mechanisms such as poly (ADP-ribosyl)ation of p53 by arsenite may be involved in p53 inactivation (Komissarova and Rossman, 2010).
Decreased cellular platinum accumulation is an important mechanism of cisplatin-resistance. Previous studies using atomic absorption spectroscopy to measure platinum levels have shown that A2780 cells accumulate approximately twofold more platinum than A2780/CP70 cells (Parker et al., 1991). Here, we used a more sensitive analytical technique (ICP-MS) and showed that A2780 cells accumulate ∼2.3-fold more platinum than A2780/CP70 cells (Fig. 5A), consistent with earlier cellular platinum measurements. In addition, we showed that A2780 and SKOV-3 cells accumulated similar level of cellular platinum, consistent with earlier findings by Johnson et al. (1997). Potential competition for the detoxification and efflux pathway by arsenic and cisplatin may increase cellular accumulation of platinum (Cepeda et al., 2007; Leslie et al., 2004). Meanwhile, hyperthermia could potentially increase membrane permeability, which will allow more drugs to enter the cells. We showed that hyperthermia ± sodium arsenite significantly enhanced cellular accumulation of platinum in A2780 cells (Fig. 5B, left panel). Combined sodium arsenite and hyperthermia also significantly enhanced platinum accumulation in A2780/CP70 cells (Fig. 5B, center panel), consistent with our in vivo findings (Muenyi et al., 2011). However, only hyperthermia increased cellular platinum accumulation in SKOV-3 cells (Fig. 5B, right panel), consistent with our cytotoxicity data (Fig. 1B). Therefore, increased cellular platinum accumulation is another mechanism by which sodium arsenite and hyperthermia sensitize wild-type p53-expressing cells to cisplatin.
Reduced platinum bound to DNA contributes to cisplatin-resistance (Parker et al., 1991). A2780/CP70 cells accumulated about twofold less platinum on their DNA compared with A2780 and SKOV-3 cells. The observed decreased accumulation could be a consequence of reduced cellular accumulation of platinum in A2780/CP70 cells (Fig. 5A). A2780 (cisplatin-sensitive) and p53-null SKOV-3 (cisplatin-resistant) cells accumulated platinum on DNA to a similar extent, contrary to previous findings by Johnson et al. (1997). The observed discrepancy in platinum bound to DNA data could be due to differences in drug exposure time and platinum determination time. Johnson et al. (1997) treated cells for 4 h and assayed for platinum at 0 (immediately after treatment) and 8 h after treatment, whereas we did a 1-h exposure and determined platinum levels at 0 and 24 h after treatment. Our data suggest that cisplatin-resistance in SKOV-3 cells is not due to decreased initial platinum bound to DNA. However, cisplatin-resistance in wild-type p53 A2780/CP70 (cisplatin-resistant) cells is due to decreased platinum bound to DNA, consistent with previous findings (Parker et al., 1991). Hyperthermia alone or combined with sodium arsenite significantly increased initial (0 h) platinum bound to DNA in all three cell lines. However, adding sodium arsenite to hyperthermia further increased platinum bound to DNA only in A2780 and A2780/CP70 cells. Platinum retention (24 h) on DNA of A2780 cells was favored by sodium arsenite, hyperthermia, or the combined treatment. Retention was favored by hyperthermia with or without sodium arsenite in A2780/CP70 and SKOV-3 cells (Fig. 6). Therefore, increased platinum bound to DNA is a mechanism by which sodium arsenite and hyperthermia sensitize wild-type p53 cells to cisplatin.
In summary, we have shown for the first time that combining sodium arsenite and hyperthermia sensitizes wild-type p53-expressing human ovarian cancer cells to cisplatin by attenuating XPC induction in response to cisplatin and by enhancing cellular and DNA bound platinum accumulation. The combination of cisplatin, sodium arsenite, and hyperthermia is more effective than cisplatin plus sodium arsenite or cisplatin plus hyperthermia. Further studies using in vivo cancer models are necessary to determine whether this combination therapy may improve clinical outcomes.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
FUNDING
This work was supported in part by the National Institutes of Health (R01 ES011314, P30 ES014443) and the National Science Foundation’s Experimental Program to Stimulate Competitive Research (EPS-0447479).
Supplementary Material
Acknowledgments
The authors thank Heather L. Miller and Xiaoqiang Xu for performing initial MTT exploratory experiments; Dr Richard Higashi and Ms Teresa Cassel at the Center for Regulatory and Environmental Analytical Metabolomics for technical support with ICP-MS analyses. Parts of this work were presented previously as follows: Clarisse S. Muenyi (2011). Mitigating Cisplatin-Resistance in Ovarian Cancer. PhD. Dissertation, University of Louisville, Louisville, KY.
References
- Cepeda V, Fuertes MA, Castilla J, Alonso C, Quevedo C, Perez JM. Biochemical mechanisms of cisplatin cytotoxicity. Anticancer Agents Med. Chem. 2007;7:3–18. doi: 10.2174/187152007779314044. [DOI] [PubMed] [Google Scholar]
- Chun YJ, Park IC, Park MJ, Woo SH, Hong SI, Chung HY, Kim TH, Lee YS, Rhee CH, Lee SJ. Enhancement of radiation response in human cervical cancer cells in vitro and in vivo by arsenic trioxide (As2O3) FEBS Lett. 2002;519:195–200. doi: 10.1016/s0014-5793(02)02765-5. [DOI] [PubMed] [Google Scholar]
- Cohen MH, Hirschfeld S, Flamm Honig S, Ibrahim A, Johnson JR, O'Leary JJ, White RM, Williams GA, Pazdur R. Drug approval summaries: arsenic trioxide, tamoxifen citrate, anastrazole, paclitaxel, bexarotene. Oncologist. 2001;6:4–11. doi: 10.1634/theoncologist.6-1-4. [DOI] [PubMed] [Google Scholar]
- Cui X, Kobayashi Y, Akashi M, Okayasu R. Metabolism and the paradoxical effects of arsenic: Carcinogenesis and anticancer. Curr. Med. Chem. 2008;15:2293–2304. doi: 10.2174/092986708785747526. [DOI] [PubMed] [Google Scholar]
- Dovern E, de Hingh IH, Verwaal VJ, van Driel WJ, Nienhuijs SW. Hyperthermic intraperitoneal chemotherapy added to the treatment of ovarian cancer. A review of achieved results and complications. Eur. J. Gynaecol. Oncol. 2010;31:256–261. [PubMed] [Google Scholar]
- Earley JN, Turchi JJ. Interrogation of nucleotide excision repair capacity: Impact on platinum-based cancer therapy. Antioxid. Redox Signal. 2011;14:2465–2477. doi: 10.1089/ars.2010.3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efeyan A, Serrano M. p53: Guardian of the genome and policeman of the oncogenes. Cell Cycle. 2007;6:1006–1010. doi: 10.4161/cc.6.9.4211. [DOI] [PubMed] [Google Scholar]
- Ford JM. Regulation of DNA damage recognition and nucleotide excision repair: Another role for p53. Mutat. Res. 2005;577:195–202. doi: 10.1016/j.mrfmmm.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Griffin RJ, Monzen H, Williams BW, Park H, Lee SH, Song CW. Arsenic trioxide induces selective tumour vascular damage via oxidative stress and increases thermosensitivity of tumours. Int. J. Hyperthermia. 2003;19:575–589. doi: 10.1080/0265673031000124316. [DOI] [PubMed] [Google Scholar]
- Hagopian GS, Mills GB, Khokhar AR, Bast RC, Jr, Siddik ZH. Expression of p53 in cisplatin-resistant ovarian cancer cell lines: Modulation with the novel platinum analogue (1R, 2R-diaminocyclohexane)(trans-diacetato)(dichloro)-platinum(IV) Clin. Cancer Res. 1999;5:655–663. [PubMed] [Google Scholar]
- Havrilesky LJ, Elbendary A, Hurteau JA, Whitaker RS, Rodriguez GC, Berchuck A. Chemotherapy-induced apoptosis in epithelial ovarian cancers. Obstet. Gynecol. 1995;85:1007–1010. doi: 10.1016/0029-7844(95)00058-y. [DOI] [PubMed] [Google Scholar]
- Helm CW, Bristow RE, Kusamura S, Baratti D, Deraco M. Hyperthermic intraperitoneal chemotherapy with and without cytoreductive surgery for epithelial ovarian cancer. J. Surg. Oncol. 2008;98:283–290. doi: 10.1002/jso.21083. [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J. Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- Johnson SW, Laub PB, Beesley JS, Ozols RF, Hamilton TC. Increased platinum-DNA damage tolerance is associated with cisplatin resistance and cross-resistance to various chemotherapeutic agents in unrelated human ovarian cancer cell lines. Cancer Res. 1997;57:850–856. [PubMed] [Google Scholar]
- Jones JC, Zhen WP, Reed E, Parker RJ, Sancar A, Bohr VA. Gene-specific formation and repair of cisplatin intrastrand adducts and interstrand cross-links in Chinese hamster ovary cells. J. Biol. Chem. 1991;266:7101–7107. [PubMed] [Google Scholar]
- Komissarova EV, Rossman TG. Arsenite-induced poly(ADP-ribosyl)ation of tumor suppressor P53 in human skin keratinocytes as a possible mechanism for carcinogenesis associated with arsenic exposure. Toxicol. Appl. Pharmacol. 2010;243:399–404. doi: 10.1016/j.taap.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong B, Huang S, Wang W, Ma D, Qu X, Jiang J, Yang X, Zhang Y, Wang B, Cui B, et al. Arsenic trioxide induces apoptosis in cisplatin-sensitive and -resistant ovarian cancer cell lines. Int. J. Gynecol. Cancer. 2005;15:872–877. doi: 10.1111/j.1525-1438.2005.00251.x. [DOI] [PubMed] [Google Scholar]
- Leslie EM, Haimeur A, Waalkes MP. Arsenic transport by the human multidrug resistance protein 1 (MRP1/ABCC1). Evidence that a tri-glutathione conjugate is required. J. Biol. Chem. 2004;279:32700–32708. doi: 10.1074/jbc.M404912200. [DOI] [PubMed] [Google Scholar]
- Lin PS, McPherson LA, Chen AY, Sage J, Ford JM. The role of the retinoblastoma/E2F1 tumor suppressor pathway in the lesion recognition step of nucleotide excision repair. DNA Repair (Amst.) 2009;8:795–802. doi: 10.1016/j.dnarep.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SY, Wen CY, Lee YJ, Lee TC. XPC silencing sensitizes glioma cells to arsenic trioxide via increased oxidative damage. Toxicol. Sci. 2010;116:183–193. doi: 10.1093/toxsci/kfq113. [DOI] [PubMed] [Google Scholar]
- Maeda H, Hori S, Nishitoh H, Ichijo H, Ogawa O, Kakehi Y, Kakizuka A. Tumor growth inhibition by arsenic trioxide (As2O3) in the orthotopic metastasis model of androgen-independent prostate cancer. Cancer Res. 2001;61:5432–5440. [PubMed] [Google Scholar]
- Martin LP, Hamilton TC, Schilder RJ. Platinum resistance: The role of DNA repair pathways. Clin. Cancer Res. 2008;14:1291–1295. doi: 10.1158/1078-0432.CCR-07-2238. [DOI] [PubMed] [Google Scholar]
- McGuire WP, Hoskins WJ, Brady MF, Kucera PR, Partridge EE, Look KY, Clarke-Pearson DL, Davidson M. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N. Engl. J. Med. 1996;334:1–6. doi: 10.1056/NEJM199601043340101. [DOI] [PubMed] [Google Scholar]
- Muenyi CS, States VA, Masters JH, Fan TW, Helm CW, States JC. Sodium arsenite and hyperthermia modulate cisplatin-DNA damage responses and enhance platinum accumulation in murine metastatic ovarian cancer xenograft after hyperthermic intraperitoneal chemotherapy (HIPEC) J. Ovarian. Res. 2011;4:9. doi: 10.1186/1757-2215-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murgo AJ. Clinical trials of arsenic trioxide in hematologic and solid tumors: Overview of the National Cancer Institute Cooperative Research and Development Studies. Oncologist. 2001;6(Suppl. 2):22–28. doi: 10.1634/theoncologist.6-suppl_2-22. [DOI] [PubMed] [Google Scholar]
- Nakagawa Y, Akao Y, Morikawa H, Hirata I, Katsu K, Naoe T, Ohishi N, Yagi K. Arsenic trioxide-induced apoptosis through oxidative stress in cells of colon cancer cell lines. Life Sci. 2002;70:2253–2269. doi: 10.1016/s0024-3205(01)01545-4. [DOI] [PubMed] [Google Scholar]
- Nakayama K, Takebayashi Y, Nakayama S, Hata K, Fujiwaki R, Fukumoto M, Miyazaki K. Prognostic value of overexpression of p53 in human ovarian carcinoma patients receiving cisplatin. Cancer Lett. 2003;192:227–235. doi: 10.1016/s0304-3835(02)00686-9. [DOI] [PubMed] [Google Scholar]
- Neher TM, Rechkunova NI, Lavrik OI, Turchi JJ. Photo-cross-linking of XPC-Rad23B to cisplatin-damaged DNA reveals contacts with both strands of the DNA duplex and spans the DNA adduct. Biochemistry. 2010;49:669–678. doi: 10.1021/bi901575h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nollen M, Ebert F, Moser J, Mullenders LH, Hartwig A, Schwerdtle T. Impact of arsenic on nucleotide excision repair: XPC function, protein level, and gene expression. Mol. Nutr. Food Res. 2009;53:572–582. doi: 10.1002/mnfr.200800480. [DOI] [PubMed] [Google Scholar]
- Okuda T, Otsuka J, Sekizawa A, Saito H, Makino R, Kushima M, Farina A, Kuwano Y, Okai T. p53 mutations and overexpression affect prognosis of ovarian endometrioid cancer but not clear cell cancer. Gynecol. Oncol. 2003;88:318–325. doi: 10.1016/s0090-8258(02)00149-x. [DOI] [PubMed] [Google Scholar]
- Olivier M, Eeles R, Hollstein M, Khan MA, Harris CC, Hainaut P. The IARC TP53 database: New online mutation analysis and recommendations to users. Hum. Mutat. 2002;19:607–614. doi: 10.1002/humu.10081. [DOI] [PubMed] [Google Scholar]
- Parker RJ, Eastman A, Bostick-Bruton F, Reed E. Acquired cisplatin resistance in human ovarian cancer cells is associated with enhanced repair of cisplatin-DNA lesions and reduced drug accumulation. J. Clin. Invest. 1991;87:772–777. doi: 10.1172/JCI115080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2011. [Google Scholar]
- Schuijer M, Berns EM. TP53 and ovarian cancer. Hum. Mutat. 2003;21:285–291. doi: 10.1002/humu.10181. [DOI] [PubMed] [Google Scholar]
- Stewart DJ. Mechanisms of resistance to cisplatin and carboplatin. Crit. Rev. Oncol. Hematol. 2007;63:12–31. doi: 10.1016/j.critrevonc.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Tang F, Liu G, He Z, Ma WY, Bode AM, Dong Z. Arsenite inhibits p53 phosphorylation, DNA binding activity, and p53 target gene p21 expression in mouse epidermal JB6 cells. Mol. Carcinog. 2006;45:861–870. doi: 10.1002/mc.20245. [DOI] [PubMed] [Google Scholar]
- Taylor BF, McNeely SC, Miller HL, States JC. Arsenite-induced mitotic death involves stress response and is independent of tubulin polymerization. Toxicol. Appl. Pharmacol. 2008;230:235–246. doi: 10.1016/j.taap.2008.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uslu R, Sanli UA, Sezgin C, Karabulut B, Terzioglu E, Omay SB, Goker E. Arsenic trioxide-mediated cytotoxicity and apoptosis in prostate and ovarian carcinoma cell lines. Clin. Cancer Res. 2000;6:4957–4964. [PubMed] [Google Scholar]
- Wang W, Qin SK, Chen BA, Chen HY. Experimental study on antitumor effect of arsenic trioxide in combination with cisplatin or doxorubicin on hepatocellular carcinoma. World J. Gastroenterol. 2001;7:702–705. doi: 10.3748/wjg.v7.i5.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Wang B. Arsenic trioxide (As(2)O(3)) inhibits peritoneal invasion of ovarian carcinoma cells in vitro and in vivo. Gynecol. Oncol. 2006;103:199–206. doi: 10.1016/j.ygyno.2006.02.037. [DOI] [PubMed] [Google Scholar]
- Zhang N, Wu ZM, McGowan E, Shi J, Hong ZB, Ding CW, Xia P, Di W. Arsenic trioxide and cisplatin synergism increase cytotoxicity in human ovarian cancer cells: Therapeutic potential for ovarian cancer. Cancer Sci. 2009;100:2459–2464. doi: 10.1111/j.1349-7006.2009.01340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.