Figure 4. Alternative splicing of neurexin regulates selectivity in neurexin-ligand interactions.
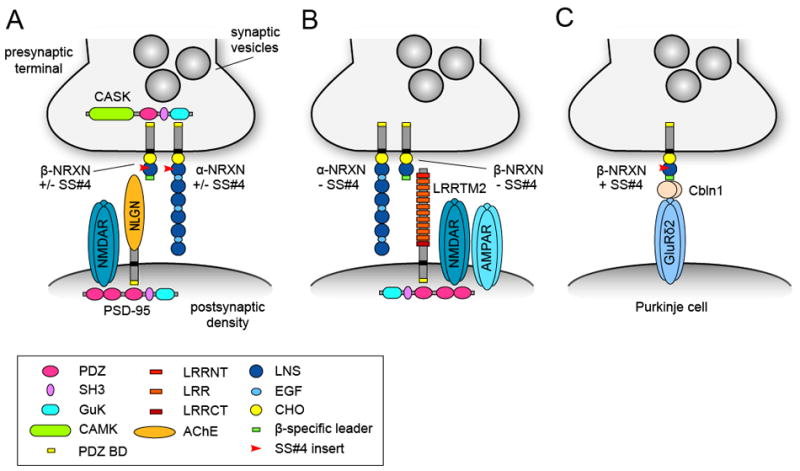
(A) Schematic drawing summarizing the trans-synaptic interaction between presynaptic α (long) and β (short) neurexins (NRXN) and its postsynaptic binding partner neuroligin (NLGN). Neurexins with or without a 30 amino acid insert at splice site #4 (SS#4) can bind neuroligins. Neurexins interact with the scaffolding molecule CASK and neuroligins interact with the scaffolding molecule PSD-95, which binds NMDAR receptors (NMDARs) via its PDZ domain. (B) Only neurexins lacking the SS#4 insert bind the postsynaptic adhesion molecule LRRTM2, which can recruit NMDARs and AMPARs. (C) SS#4 containing neurexins in cerebellar granule cells form a synapse-specific trans-synaptic adhesion complex with the secreted cerebellin precursor protein 1 (Cbln1) and the postsynaptic GluRδ2 receptor on Purkinje cell dendritic spines. GuK, guanylate kinase domain; CaMK, Ca2+/calmodulin-dependent kinase domain; LRR, leucine-rich repeat; LRRNT and LRRCT, N-terminal and C-terminal LRR flanking domains; PDZ BD, PDZ binding domain; AChE, acetylcholinesterase homology domain; LNS, laminin/neurexin/sex-hormone-binding protein domain; EGF, epidermal growth factor-like domain; CHO, carbohydrate attachment sequence.
