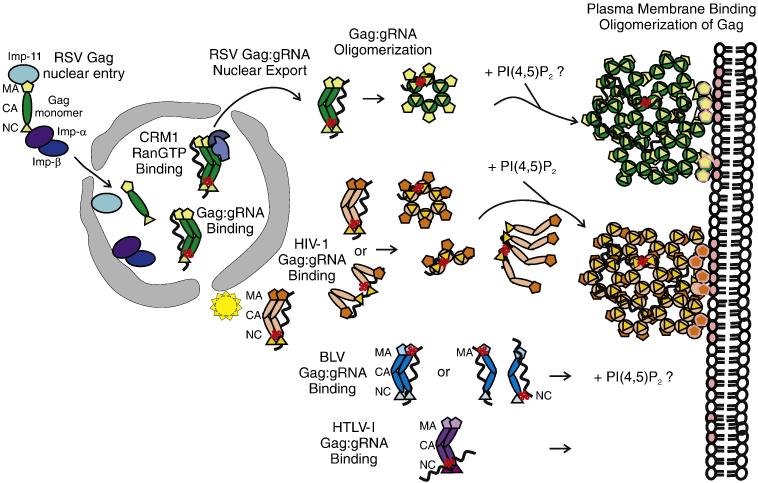
Fig. 2. Model illustrating the role of the Gag MA domain in regulating gRNA binding, subcellular trafficking, genome encapsidation and particle assembly.
After synthesis in the cytoplasm, Gag proteins destined to bind the gRNA for packaging are transported to specific subcellular locations. The MA domain of Gag is represented by pentagons, CA by ovals, and NC by triangles. The gRNA is a wavy black line and the ψ sequence is depicted as a red cloverleaf. For RSV Gag (green), NLSs in the MA and NC domains interact with host import factors importin-11 and the importin-α/β complex to direct Gag into the nucleus where Gag interacts with the ψ sequence on the gRNA. RSV Gag:gRNA binding induces a conformation change in RSV Gag that promotes binding to CRM1:RanGTP, facilitating nuclear export. RSV Gag forms oligomers that are transported to the plasma membrane, possibly through an interaction with the phosphoinositol PIP(4,5)2 (denoted by pink ovals in the inner leaflet of the lipid bilayer). A discontinuous hexameric lattice of RSV Gag proteins bound to gRNA assembles at the membrane, encapsidating the genome into the assembling particle. For HIV (orange), Gag interacts with its gRNA at a pericentriolar location (illustrated as a yellow star) or in the cytoplasm, inducing Gag dimer formation. The model illustrates the MA and NC domains interacting with gRNA in an extended conformation (top) or in a folded conformation (bottom), with the NC domain binding to ψ and the MA domain binding to the gRNA at a different location. It is possible that the MA domain is bound to a cellular RNA rather than to the gRNA. HIV-1 Gag:gRNA oligomers form and are transported toward the periphery of the cell. Upon binding to PIP(4,5)2, the MA domain releases the gRNA and Gag adopts an extended conformation with MA facing the membrane and NC binding to the gRNA. The hexamers of Gag associate with the plasma membrane and assemble into a hexameric lattice. For BLV (blue), the site of Gag:gRNA complex formation is not known. The MA domain of BLV Gag is shown binding to the ψ sequence, although the NC domain also contacts the gRNA through nonspecific interactions. Further details regarding the mechanism of BLV Gag assembly are not well understood. For HTLV-I, the model depicts the ψ sequence on the gRNA binding to the NC domain of Gag because it is not known whether MA plays a role in genome encapsidation. It has been shown that nucleic acid binding does not influence membrane binding, and PIP(4,5)2 is not required for membrane targeting119