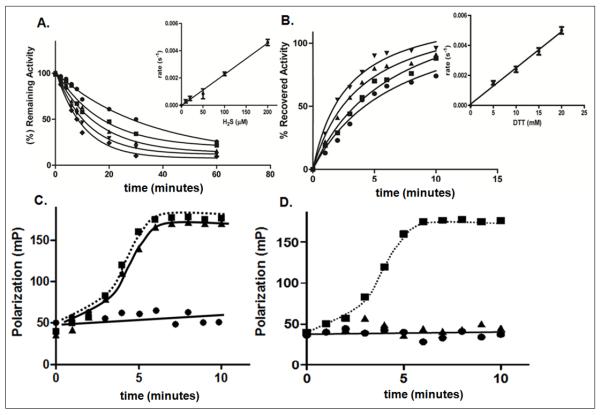
Figure 1. Redox-dependent inactivation and reactivation of PTP1B.
A. Time dependent inactivation of PTP1B by H2S. The phosphatase activity of PTP1B was monitored in the presence of the following concentrations of H2S, 10 μM (●), 20 μM (■), 50 μM (▲), 100 μM (▼), 200 μM (◆). Inset, the concentration dependence of the rate of inactivation was used to derive the second order rate constant, 22.4 ± 1.8 M−1s−1.
B. Time dependent reactivation of PTP1B by DTT. The following concentrations of DTT were used 5 mM (●), 10 mM (■), 15 mM (▲), 20 mM (▼). Inset, the concentration dependence of the rate of reactivation was used to derive the second order rate constant, 0.24 ± 0.1 M−1s−1. Data are representative of three independent determinations.
C and D. Binding of fluorescently-labeled phosphopeptide substrate (5′-FAM-ENDpYINASL) to WT (●), C215S (■), or D181A (▲) mutant forms of PTP1B. The change in fluorescence polarization is plotted as a function of time. The assays were performed in the absence of H2S (C) or following incubation with 100 μM H2S for 10 min (D).