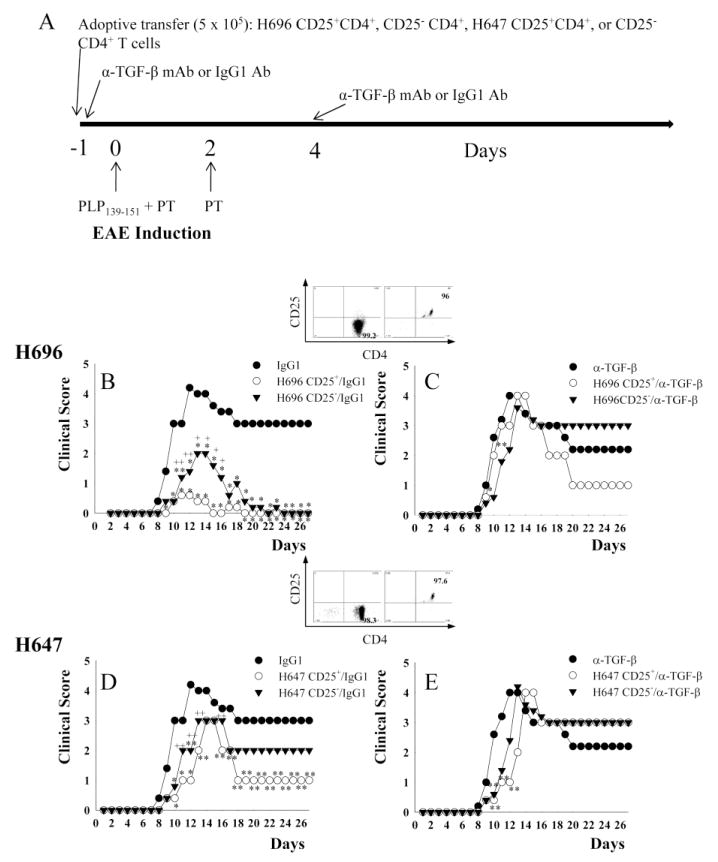
Fig. 1.
Adoptive transfer of Salmonella-CFA/I-induced CD25+CD4+ T cells confers protection against EAE, but is abrogated upon in vivo neutralization of TGF-β. CD25+CD4+ and CD25−CD4+ T cells obtained from mice previously orally immunized with Salmonella-CFA/I (strain H696) or Salmonella vector (strain H647) were adoptively transferred into naive SJL mice. A, Schematic of adoptive transfer, EAE challenge with PLP139–151, and in vivo neutralization with anti-TGF-β mAb or isotype MOPC 21 IgG1 control Ab is shown; PT, pertussis toxin. EAE clinical scores following adoptive transfer, EAE challenge, and in vivo treatment with IgG1 Ab or anti-TGF-β mAb of recipients given B,C, CD25+CD4+ and CD25− CD4+ T cells (purity plots in inset) from H696-immunized mice; and D, E, CD25+CD4+ and CD25− CD4+ T cells (purity plots in inset) from H647-immunized mice are shown. Data depict mean clinical scores combined from three experiments: *, p < 0.001; **, p < 0.05 versus PBS/IgG1or PBS/α-TGF-β mAb-treated mice; +, p < 0.001; ++, p < 0.05 for CD25+ versus CD25−.