Figure 2.
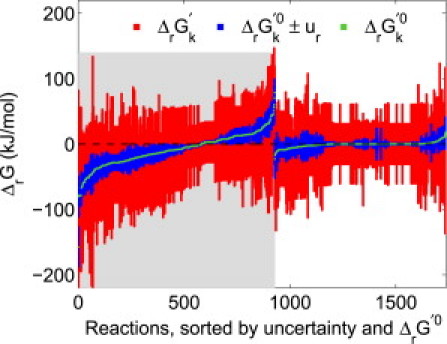
Standard transformed reaction Gibbs energy (ΔrG k′0, green) with uncertainty (ur, blue), as well as feasible ranges of transformed reaction Gibbs energy (ΔrGk′, red), for all quantitatively reversible reactions in Recon 1. Approximately half of the reactions were reversible due to the broad, generic metabolite concentration range (10−7–10−2 M) used in our analysis (shaded region). A more precise prediction of the directionality of these reactions could be made upon increased availability of compartmentally resolved quantitative metabolomic data. The other half of quantitatively reversible reactions were reversible because the range ΔrG k′0 ± ur included 0 kJ/mol (unshaded region). Availability of more precise thermodynamic data would further aid prediction of the directionality of these reactions.
